D-Luciferin
Synonym(s):(S)-2-(6-Hydroxy-2-benzothiazolyl)-2-thiazoline-4-carboxylic acid;D -Luciferin;4,5-Dihydro-2-(6-hydroxy-2-benzothiazolyl)-4-thiazolecarboxylic acid;Firefly Luciferin
- CAS NO.:2591-17-5
- Empirical Formula: C11H8N2O3S2
- Molecular Weight: 280.32
- MDL number: MFCD00042929
- EINECS: 219-981-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
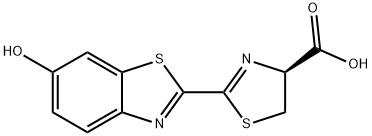
What is D-Luciferin?
Description
D-Luciferin is a popular bioluminescent substrate of luciferase in the presence of ATP, used in luciferase-based bioluminescence imaging and cell-based high-throughput screening applications. In an immunocompetent mouse model of ovarian cancer, the use of D-luciferin substrate and firefly luciferase preserves tumour-host immune interactions since bioluminescent imaging is a more sensitive indication of tumour growth than weight gain.
Chemical properties
off-white to light yellow powder
The Uses of D-Luciferin
D-Luciferin has been used in determining luciferase activity by luciferase assay in mice and Arabidopsis thaliana seeds. It has also been used in measuring bioluminescence in neonatal rat ventricular cardiomyocytes.
The Uses of D-Luciferin
In the assay of ATP.
The Uses of D-Luciferin
D-Luciferin has been used in the preparation of the reaction mixture for nitric oxide determination by minescence measurement. It has also been used in the preparation of D-luciferin stock solution for luciferin assays.
What are the applications of Application
D-Luciferin is a chemiluminescent luciferase substrate
Definition
ChEBI: A 1,3-thiazolemonocarboxylic acid consisting of 3,5-dihydrothiophene-4-carboxylic acid having a 6-hydroxybenzothiazol-2-yl group at the 2-position.
General Description
D-Luciferin is a small molecule present abundantly in bioluminescent organisms. This molecule is sensitive to oxygen and light.
Biochem/physiol Actions
D-Luciferin uptake within the cells is regulated by the adenosine triphosphate (ATP)-binding cassette (ABC) transporter inhibitors like ATP-binding cassette transporter G2 (ABCG2)/ breast cancer resistance protein (BCRP) inhibitor fumitremorgin C. This substrate is oxidized by firefly luciferase that results in emitting a photon, which has the capability of passing through living tissues.
Purification Methods
D-Luciferin crystallises as pale yellow needles from H2O, or MeOH (83mg/7mL). It has UV max at 263 and 327nm (log 3.88 and 4.27) in 95% EtOH. The Na salt has a solubility of 4mg in 1 mL of 0.05M glycine. [White et al. J Am Chem Soc 83 2402 1961, 85 337 1963, UV and IR: Bitler & McElroy Arch Biochem 72 358 1957, Review: Cormier et al. Fortschr Chem Org Naturst 30 1 1973, Beilstein 27 III/IV 8934.]
Properties of D-Luciferin
| Melting point: | 200-204 °C |
| Boiling point: | 587.6±60.0 °C(Predicted) |
| alpha | D22 -36° (c = 1.2 in DMF) |
| Density | 1.4916 (rough estimate) |
| refractive index | 1.5650 (estimate) |
| storage temp. | -20°C |
| solubility | DMF:30.0(Max Conc. mg/mL);107.02(Max Conc. mM) DMSO:35.33(Max Conc. mg/mL);126.05(Max Conc. mM) |
| pka | 8.31±0.40(Predicted) |
| form | Powder |
| color | Off-white to light yellow |
| λmax | 360nm(H2O)(lit.) |
| Merck | 14,4086 |
| BRN | 30484 |
| InChI | InChI=1S/C11H8N2O3S2/c14-5-1-2-6-8(3-5)18-10(12-6)9-13-7(4-17-9)11(15)16/h1-3,7,14H,4H2,(H,15,16)/t7-/m1/s1 |
Safety information for D-Luciferin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for D-Luciferin
| InChIKey | BJGNCJDXODQBOB-SSDOTTSWSA-N |
| SMILES | S1C[C@H](C(O)=O)N=C1C1=NC2=CC=C(O)C=C2S1 |
D-Luciferin manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 D-Luciferin 95% CAS 2591-17-5View Details
D-Luciferin 95% CAS 2591-17-5View Details
2591-17-5 -
 D-Luciferin ex. Firefly CAS 2591-17-5View Details
D-Luciferin ex. Firefly CAS 2591-17-5View Details
2591-17-5 -
![D-(-)-Luciferin [Chemiluminescence Reagent] CAS 2591-17-5](https://img.chemicalbook.in//Content/image/CP5.jpg) D-(-)-Luciferin [Chemiluminescence Reagent] CAS 2591-17-5View Details
D-(-)-Luciferin [Chemiluminescence Reagent] CAS 2591-17-5View Details
2591-17-5 -
 D-Luciferin 98% (HPLC) CAS 2591-17-5View Details
D-Luciferin 98% (HPLC) CAS 2591-17-5View Details
2591-17-5 -
 D-Luciferin CAS 2591-17-5View Details
D-Luciferin CAS 2591-17-5View Details
2591-17-5 -
 D-Luciferin CAS 2591-17-5View Details
D-Luciferin CAS 2591-17-5View Details
2591-17-5 -
 2591-17-5 D-Luciferin 98%View Details
2591-17-5 D-Luciferin 98%View Details
2591-17-5 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1