D(+)-Carvone
Synonym(s):(+)-Carvone;(S)-(+)-Carvone;(S)-5-Isopropenyl-2-methyl-2-cyclohexenone;(S)-(+)-5-Isopropenyl-2-methyl-2-cyclohexenone, (S)-(+)-p-Mentha-6,8-dien-2-one;(S)-(+)-Carvone
- CAS NO.:2244-16-8
- Empirical Formula: C10H14O
- Molecular Weight: 150.22
- MDL number: MFCD00062997
- EINECS: 218-827-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
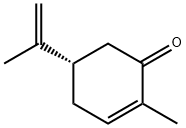
What is D(+)-Carvone?
Description
The nose knows. (S)-(+)-carvone (shown here), a naturally occurring compound found in caraway seeds and mandarin orange peel oil, smells like caraway. Its mirror image, (R)-(–)-carvone, is extracted from spearmint and kuromoji oils and smells like spearmint. Gingergrass oil contains a mixture of both enantiomers.
Description
Carvone is a pale yellow to white clear liquid.Molecular weight=150.22; Boiling point=230℃; Flashpoint=93℃. Hazard Identification (based on NFPA 704 MRating System): Health 2, Flammability 2, Reactivity 0.
Chemical properties
D(+)-Carvone is a clear colorless or pale yellow liquid. Darkens upon exposure to air and daylight. Sp.Gr. 0.97. BP. 230℃. Insoluble in water, soluble in alcohol and oils. Warm-herbaceous, breadlike, spicy and slightly floral odor, reminiscent of Caraway seed or Dill seed. Warm, sweet, spicy - herbaceous, breadlike taste.
Chemical properties
Caravone occurs in different forms. l-Carvone exhibits odor of spearmint, while d-carvone exhibits odor reminiscent of caraway.
Occurrence
The optically active and inactive forms have been reported among the constituents of about 70 essential oils. The dextro form is present in carvi, Antheum graveolens, Antheum sowa, Lippia carviodora, Mentha arvensis, etc. The levo form is present in Metha vifidis var. crispa, Mentha longifolia from South Africa, Eucalyptus globules and several mint species. The racemic form is present in ginger grass, Litsea gutalemaleusis, lavender and Artemisia ferganensis. Reported found in citrus oil and juice (lemon, lime, orange), celery seed, anise, clove, coriander seed, calamus, caraway herb and dill seed.
The Uses of D(+)-Carvone
(S)-(+)-Carvone can be used as a starting material to synthesize:
- (-)-Samaderine Y, a pentacyclic quassinoid.
- (-)-Ambrox, a terpenoid responsible for the odor of ambergris.
- 3β-Acetoxydrimenin (a sesquiterpene) via conjugated addition of potassium cyanide followed by base catalyzed Robinson annulation reaction.
- Thapsigargin family members such as trilobolide, nortrilobolide, and thapsivillosin F.
The Uses of D(+)-Carvone
D(+)-Carvone is occasionally used in perfumes, but the lacvo-Carvone is often preferred here. It is used in flavor compositions as a fortifier for Caraway seed oil or Dill oil seed (in which oils this ketone is a major component). Since this isomer is rarely offered in the synthetic form, it has not become as popular as the laevo-Carvone. The use of Caraway-Dill flavor is confined to a few, although by volume very large, commercial products (Bread, Pickles. Spices, etc.).
What are the applications of Application
(+)-Carvone is a terpene standard for gas chromatography
Definition
ChEBI: A carvone having (S) configuration.
Aroma threshold values
Detection: d-Carvone: 6.7 to 820 ppb; l-carvone: 2.7 to 600 ppb
General Description
Pale yellow or colorless liquid.
Air & Water Reactions
May be sensitive to prolonged exposure to light and air. Insoluble in water.
Reactivity Profile
D(+)-Carvone may be sensitive to prolonged exposure to light and air. Incompatible with strong oxidizing agents and strong reducing agents
Fire Hazard
D(+)-Carvone is combustible.
Flammability and Explosibility
Not classified
Safety Profile
Poison by ingestion and skin contact. A skin irritant. When heated to decomposition it emits acrid smoke and irritating fumes.
Synthesis
Carvone occurs in the dextro, levo and racemic form; l-carvone can be isolated from the essential oil of spearmint or is commercially synthesized from d-limonene; d-carvone is usually prepared by fractional distillation of oil of caraway, also from dillseed and dillweed oils, but this type differs in odor and flavors.
Potential Exposure
Carvone is found in various natural oils, including caraway and dillseed; mandarin peel and spearmint oils. A food additive, it is used in flavoring liqueurs; in perfumes and soaps.
First aid
Skin Contact: Flood all areas of body that have contacted the substance with water. Do not wait to remove contaminated clothing; do it under the water stream. Use soap tohelp assure removal. Isolate contaminated clothing whenremoved to prevent contact by others. Eye Contact: Removeany contact lenses at once. Flush eyes well with copious quantities of water or normal saline for at least 20-30 min. Seekmedical attention. Inhalation: Leave contaminated area immediately; breathe fresh air. Proper respiratory protection must besupplied to all rescuers. If coughing, difficult breathing, or anyother symptoms develop, seek medical attention at once, evenif symptoms develop many hours after exposure. Ingestion: Ifconvulsions are not present, give a glass or two of water ormilk to dilute the substance. Assure that the person’s airway isunobstructed and contact a hospital or poison center immediately for advice on whether or not to induce vomiting.
storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Store in a cool, dry place or in a refrigerator
Shipping
UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Properties of D(+)-Carvone
| Melting point: | 88.9°C |
| Boiling point: | 96-98 °C/10 mmHg (lit.) |
| alpha | 57 º (c=neat) |
| Density | 0.96 g/mL at 25 °C (lit.) |
| vapor pressure | 20.665hPa at 25℃ |
| FEMA | 2249 | CARVONE |
| refractive index | n |
| Flash point: | 204 °F |
| storage temp. | Store below +30°C. |
| solubility | 1300mg/l |
| form | Liquid |
| color | Clear colorless to pale yellow |
| Odor | at 100.00 %. spice mint bread caraway |
| optical activity | [α]23/D +55.0±7°, neat |
| Water Solubility | <0.1 g/100 mL at 25 ºC |
| Merck | 14,1874 |
| JECFA Number | 380 |
| BRN | 2042970 |
| Dielectric constant | 11.0(22℃) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, strong reducing agents. |
| CAS DataBase Reference | 2244-16-8(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Cyclohexen-1-one, 2-methyl-5-(1-methylethenyl)-, (S)-(2244-16-8) |
| EPA Substance Registry System | D-Carvone (2244-16-8) |
Safety information for D(+)-Carvone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for D(+)-Carvone
D(+)-Carvone manufacturer
Indenta Chemicals (India) Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 D-Carvone 98%View Details
D-Carvone 98%View Details -
 (S)-(+)-Carvone CAS 2244-16-8View Details
(S)-(+)-Carvone CAS 2244-16-8View Details
2244-16-8 -
 D(+)-Carvone 98% (GC) CAS 2244-16-8View Details
D(+)-Carvone 98% (GC) CAS 2244-16-8View Details
2244-16-8 -
 (S)-(+)-Carvone CAS 2244-16-8View Details
(S)-(+)-Carvone CAS 2244-16-8View Details
2244-16-8 -
 (+)-Carvone CAS 2244-16-8View Details
(+)-Carvone CAS 2244-16-8View Details
2244-16-8 -
 (S)-(+)-Carvone CAS 2244-16-8View Details
(S)-(+)-Carvone CAS 2244-16-8View Details
2244-16-8 -
 (S)-(+)-Carvone CAS 2244-16-8View Details
(S)-(+)-Carvone CAS 2244-16-8View Details
2244-16-8 -
 (+)-Carvone CAS 2244-16-8View Details
(+)-Carvone CAS 2244-16-8View Details
2244-16-8