CYTOCHALASIN D
Synonym(s):Cytochalasin D, Zygosporium mansonii - CAS 22144-77-0 - Calbiochem;InSolution Cytochalasin D, Zygosporium mansonii - CAS 22144-77-0 - Calbiochem;Lygosporin A, Zygosporin A, NSC 209835;Zygosporin A
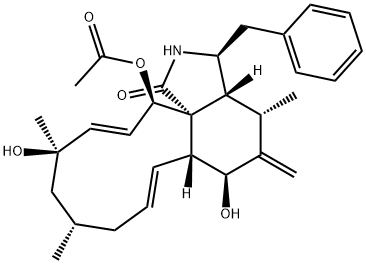
- CAS NO.:22144-77-0
- Empirical Formula: C30H37NO6
- Molecular Weight: 507.62
- MDL number: MFCD00077706
- EINECS: 244-804-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
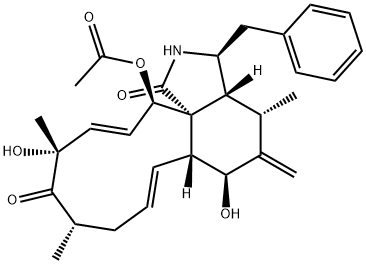
What is CYTOCHALASIN D?
Description
Cytochalasin D (22144-77-0) is a potent inhibitor of actin polymerization which also causes the disruption of actin filaments. More potent that cytochalasin B (10-fold) and does not inhibit monosaccharide transport across cell membranes. Disruption of actin microfilaments leads to activation of p53. Cell permeable
Chemical properties
Powder
The Uses of CYTOCHALASIN D
Cytochalasin D acts as an anti-hypertensive drug acting on human intestinal epithelial caco-2 cells. Also has activity in HMG-CoA reductase inhibitors used in the lowering of cholesterol. Actin inhib itor, actin polymerization inhibitor affecting cell movement, growth, phagocytosis and even secretion.
The Uses of CYTOCHALASIN D
Cytochalasin D is the most studied of the cytochalasins. Like most of the members of this mycotoxin class, cytochalasin D exhibits potent inhibition of actin filament function leading to cell death by apoptosis. This led to early investigation of the metabolite as an antitumour agent. Cytochalasin D has become one of the standard cellular probes for investigating the role of actin in cell biology.
The Uses of CYTOCHALASIN D
A cell cycle arresting compound used in actin polymerization studies and cytological research
What are the applications of Application
Cytochalasin D is a cell cycle arresting compound used in actin polymerization studies and cytological research
Definition
ChEBI: An organic heterotricyclic compound that is a mycotoxin produced by Helminthosporium and other moulds which is cell permeable and a potent inhibitor of actin polymerisation and DNA synthesis.
General Description
Needles or fluffy white powder.
Reactivity Profile
CYTOCHALASIN D may be sensitive to exposure to heat. CYTOCHALASIN D can react with strong oxidizing agents, strong acids and strong bases. .
Fire Hazard
Flash point data for CYTOCHALASIN D are not available; however, CYTOCHALASIN D is probably combustible.
Biological Activity
Potent disruptor of actin filament function. Alters tight junction permeability. Unlike cytochalasin B, does not inhibit monosaccharide transport across the plasma membrane.
Biochem/physiol Actions
Cell permeable fungal toxin; potent inhibitor of actin polymerization. Disrupts actin microfilaments and activates the p53-dependent pathways causing arrest of the cell cycle at the G1-S transition. Inhibits smooth muscle contraction. Inhibits insulin-stimulated, but not basal, transport of glucose.
Safety Profile
Poison by ingestion, subcutaneous, and intraperitoneal routes. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Storage
Store at -20°C
References
1)) Goddetteand et al. (1986), Actin polymerization. The mechanism of action of cytochalasin D; J. Biol. Chem., 261 15974 2) Rubtsova et al. (1998), Disruption of actin microfilaments by cytochalasin D leads to activation of p53; FEBS Lett., 430 353
Properties of CYTOCHALASIN D
| Melting point: | 255-260°C |
| Boiling point: | 595.84°C (rough estimate) |
| Density | 1.1764 (rough estimate) |
| refractive index | 1.6310 (estimate) |
| Flash point: | 87℃ |
| storage temp. | −20°C |
| solubility | DMSO: soluble |
| form | White solid |
| pka | 11.95±0.70(Predicted) |
| color | White |
| BRN | 1632828 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
| EPA Substance Registry System | Cytochalasin D (22144-77-0) |
Safety information for CYTOCHALASIN D
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for CYTOCHALASIN D
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 Cytochalasin D, from zygosporium mansonii CAS 22144-77-0View Details
Cytochalasin D, from zygosporium mansonii CAS 22144-77-0View Details
22144-77-0 -
 Cytochalasin D, Zygosporium mansonii CAS 22144-77-0View Details
Cytochalasin D, Zygosporium mansonii CAS 22144-77-0View Details
22144-77-0 -
 Cytochalasin D CAS 22144-77-0View Details
Cytochalasin D CAS 22144-77-0View Details
22144-77-0 -
 Cytochalasin D CAS 22144-77-0View Details
Cytochalasin D CAS 22144-77-0View Details
22144-77-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
