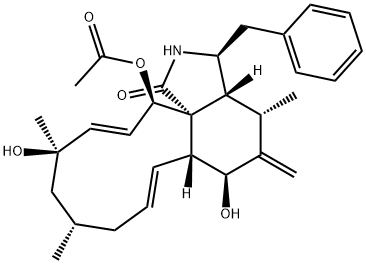
CYTOCHALASIN A
- CAS NO.:14110-64-6
- Empirical Formula: C29H35NO5
- Molecular Weight: 477.59
- MDL number: MFCD00005935
- EINECS: 237-964-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02

What is CYTOCHALASIN A?
Description
The cytochalasins are cell-
Chemical properties
white powder
The Uses of CYTOCHALASIN A
Cytochalasin A is one of a family of potent mycotoxins produced by several species of fungi. All members of the class exhibit profound effects on cytoskeletal proteins, giving rise to pronounced morphogenic activity in animals and plants. Like most cytochalsins, cytochalasin A exhibits potent inhibition of actin filament function leading to cell death by apoptosis, and displays a broad range of resultant cellular actions. Despite the common mode of action, there is evidence that individual members of the class display diverse selectivity. Specifically, cytochalasin A is one of the few cytochalasins exhibiting activity against HIV-1 protease.
The Uses of CYTOCHALASIN A
Cytochalasin A binds to the glucose transporter and inhibit monosaccharide transport across the plasma membrane. Preventing growth and sugar uptake in a Saccharomyces strain.
What are the applications of Application
Cytochalasin A is an HIV-1 protease inhibitor, antibiotic, glucose transport inhibitors, and actin polymerization inhibitor
Definition
ChEBI: Cytochalasin A is a cytochalasin.
Biochem/physiol Actions
Cytochalasin A reacts with sulfhydryls, and inhibits growth and glucose transport in Saccharomyces. Cytochalasin A is an oxidizing analog of cytochalsin B.
Properties of CYTOCHALASIN A
| Melting point: | 190-191 °C |
| Boiling point: | 725.1±60.0 °C(Predicted) |
| Density | 1.20±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | ethanol: 20 mg/mL, clear, colorless |
| pka | 13.54±0.70(Predicted) |
| form | Powder |
| color | White |
| Water Solubility | Soluble in DMF, DMSO, ethanol and acetone. Insoluble in water. |
| Sensitive | Light Sensitive |
| BRN | 949521 |
| EPA Substance Registry System | Cytochalasin A (14110-64-6) |
Safety information for CYTOCHALASIN A
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H361:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for CYTOCHALASIN A
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran



You may like
-
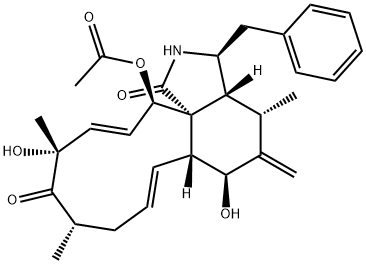
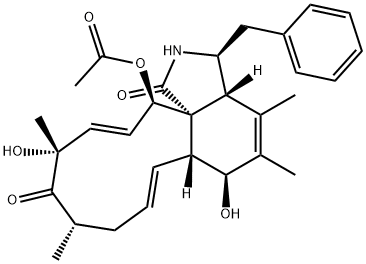
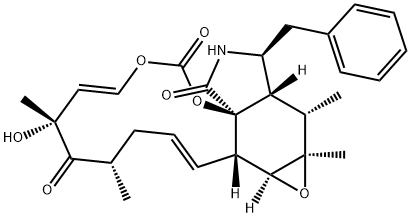
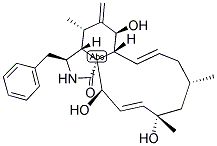
 Cytochalasin A, from drechslera dematioidea CAS 14110-64-6View Details
Cytochalasin A, from drechslera dematioidea CAS 14110-64-6View Details
14110-64-6 -
 Cytochalasin A from Drechslera dematioidea CAS 14110-64-6View Details
Cytochalasin A from Drechslera dematioidea CAS 14110-64-6View Details
14110-64-6 -
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
