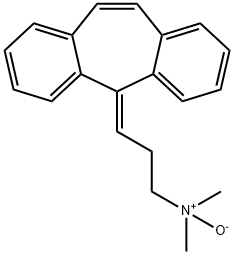
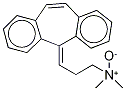
CYCLOBENZAPRINE HCL
- CAS NO.:303-53-7
- Empirical Formula: C20H21N
- Molecular Weight: 275.39
- MDL number: MFCD00867680
- EINECS: 206-145-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-17 14:56:35
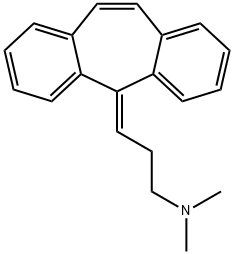
What is CYCLOBENZAPRINE HCL?
Absorption
The oral bioavailability of cyclobenzaprine has been estimated to be between 0.33 and 0.55. Cmax is between 5-35 ng/mL and is achieved after 4 hours (Tmax). AUC over an 8 hour dosing interval was reported to be approximately 177 ng.hr/mL.
Toxicity
The oral LD50 of cyclobenzaprine in mice and rats is 338 mg/kg and 425 mg/kg, respectively. Signs of overdose may develop rapidly after ingestion and commonly include significant drowsiness and tachycardia, with less common manifestations including tremor, agitation, ataxia, GI upset, and other CNS effects such as confusion and hallucinations. Potentially critical manifestations, though rare, include cardiac arrest or dysrhythmias, severe hypotension, seizures, and neuroleptic malignant syndrome.
As the management of cyclobenzaprine overdose is complex and ever-changing, it is recommended that a poison control center be consulted prior to treatment. Typical management involves gastrointestinal decontamination, close cardiac monitoring, and monitoring for signs of CNS or respiratory depression. As cyclobenzaprine exists in relatively low concentrations in plasma, monitoring of drug plasma levels should not guide management and dialysis is likely of no value.
Description
Cyclobenzaprine is structurally similar to tricyclic antidepressants. It acts at the brain stem level.
Originator
Flexeril,Merck Sharp andDohme,US,1977
The Uses of CYCLOBENZAPRINE HCL
Relaxant (muscle).
The Uses of CYCLOBENZAPRINE HCL
It is used as an adjuvant agent for relieving muscle spasms associated with severe diseased conditions of the muscle. A synonym of this drug is flexeril.
Indications
Cyclobenzaprine is indicated as a short-term (2-3 weeks) adjunct therapy, along with rest and physical therapy, for relief of muscle spasm associated with acute, painful musculoskeletal conditions. It has not been found effective in the treatment of spasticity originating from cerebral or spinal cord disease, or spasticity in children with cerebral palsy. Cyclobenzaprine is also occasionally used off-label for reducing pain and sleep disturbances in patients with fibromyalgia.
Background
Cyclobenzaprine, a centrally-acting muscle relaxant, was first synthesized in 1961 and has been available for human use since 1977. It was initially studied for use as antidepressant given its structural similarity to tricyclic antidepressants - it differs from Amitriptyline by only a single double bond. Since its approval, it has remained relatively popular as an adjunctive, short-term treatment for acute skeletal muscle spasms secondary to musculoskeletal injury.
Definition
ChEBI: 5-Methylidene-5H-dibenzo[a,d]cycloheptene in which one of the hydrogens of the methylidene group is substituted by a 2-(dimethylamino)ethyl group. A centrally acting skeletal muscle relaxant, it is used as its h drochloride salt in the symptomatic treatment of painful muscle spasm.
Manufacturing Process
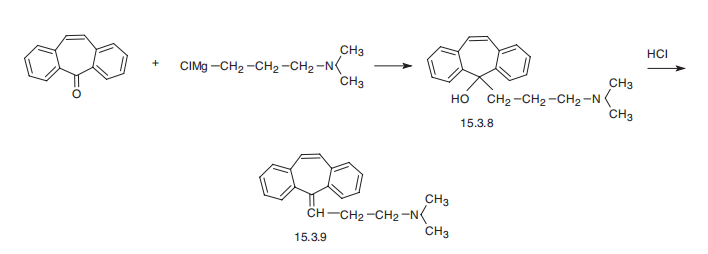
In an initial step, dibenzo [a,d]cyclohepten-5-one is reacted with the Grignardreagent of 3-dimethylaminopropyl chloride and hydrolyzed to give 5-(3-dimethylaminopropyl)-dibenzo[a,d][1,4]cycloheptatriene-5-ol. Then 13 g ofthat material, 40 ml of hydrochloric acid, and 135 ml of glacial acetic acid isrefluxed for 3? hours. The solution is then evaporated to dryness in vacuoand added to ice water which is then rendered basic by addition of ammoniumhydroxide solution. Extraction of the basic solution with chloroform andremoval of the solvent from the dried chloroform extracts yields the crudeproduct which when distilled in vacuo yields essentially pure 5-(3-dimethylaminopropylidene)-dibenzo[a,d][1,4]cycloheptatriene, BP 173°C to177°C at 1.0 mm.
brand name
Flexeril (McNeil).
Therapeutic Function
Muscle relaxant
Pharmacokinetics
Cyclobenzaprine is a skeletal muscle relaxant that works on areas of the brainstem to reduce skeletal muscle spasm, though its exact pharmacodynamic behaviour is currently unclear. Despite its long half-life, it is relatively short-acting with a typical duration of action of 4-6 hours. Cyclobenzaprine has been reported to contribute to the development of serotonin syndrome when used in combination with other serotonergic medications. Symptoms of serotonin syndrome may include autonomic instability, changes to mental status, neuromuscular abnormalities, or gastrointestinal symptoms - treatment with cyclobenzaprine should be discontinued immediately if any of these reactions occur during therapy.
Synthesis
Cyclobenzaprine, N,N-dimethyl-3-(dibenzo[a,d]cyclohepten-5-ylidene) propylamine (15.3.9), is synthesized by reacting 5H-dibenzo[a,d]cyclohepten-5-one with 3-dimethylaminopropylmagnesium chloride and subsequent dehydration of the resulting carbinol (15.3.8) in acidic conditions into cyclobenzaprine (15.3.9).
Metabolism
Cyclobenzaprine is extensively metabolized in the liver via both oxidative and conjugative pathways. Oxidative metabolism, mainly N-demethylation, is catalyzed primarily by CYP3A4 and CYP1A2 (with CYP2D6 implicated to a lesser extent) and is responsible for the major metabolite desmethylcyclobenzaprine. Cyclobenzaprine also undergoes N-glucuronidation in the liver catalyzed by UGT1A4 and UGT2B10, and has been shown to undergo enterohepatic circulation.
Properties of CYCLOBENZAPRINE HCL
| Boiling point: | bp1 175-180° |
| Density | 0.9504 (rough estimate) |
| refractive index | 1.7500 (estimate) |
| pka | pKa 8.47 (Uncertain) |
| NIST Chemistry Reference | Cyclobenzaprine(303-53-7) |
Safety information for CYCLOBENZAPRINE HCL
Computed Descriptors for CYCLOBENZAPRINE HCL
CYCLOBENZAPRINE HCL manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran



You may like
-
 303-53-7 Cyclobenzaprine 98%View Details
303-53-7 Cyclobenzaprine 98%View Details
303-53-7 -
 303-53-7 99%View Details
303-53-7 99%View Details
303-53-7 -
 Cyclobenzaprine 99%View Details
Cyclobenzaprine 99%View Details -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
