Cyproheptadine
- CAS NO.:129-03-3
- Empirical Formula: C21H21N
- Molecular Weight: 287.4
- MDL number: MFCD00242817
- EINECS: 204-928-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-05 14:33:18

What is Cyproheptadine?
Absorption
A single study examining the difference in absorption of orally administered versus sublingually administered cyproheptadine in five healthy males demonstrated a mean Cmax of 30.0 mcg/L and 4.0 mcg/L, respectively, and a mean AUC of 209 mcg.h/L and 25 mcg.h/L, respectively. The Tmax of orally and sublingually administered cyproheptadine was 4 hours and 9.6 hours, respectively.
Toxicity
Overdosage with cyproheptadine is likely to result in significant sedation - although paradoxical stimulation has been noted in pediatric patients - and anticholinergic adverse effects such as dry mouth and flushing. Most patients appear to recover without incident, as a review of cyproheptadine overdose cases in Hong Kong found the majority of patients had no or mild symptoms following intentional overdose.
In the event of overdosage with cyproheptadine, prescribing information recommends the induction of vomiting (if it has not occurred spontaneously) using syrup of ipecac. Gastric lavage and activated charcoal may also be considered. Vasopressors may be used to treat hypotension and intravenous physostigmine salicylate may be considered for the treatment of significant CNS symptoms depending on the clinical picture.
Description
Cyproheptadine has antianaphylactic activity that is associated with its ability to slow down the release of histamine and other mediators from fat cells.
Originator
Periactin,Merck Sharp and Dohme,US,1961
The Uses of Cyproheptadine
Antihistaminic; antipruritic.
The Uses of Cyproheptadine
It is mainly used for treating bronchial asthma attacks, allergic bronchitis, rhinitis, and allergic skin reactions as well as in adjuvant therapy for anaphylactic reactions. Synonyms of this drug are periactin and vimicon.
Indications
In the US, prescription cyproheptadine is indicated for the treatment of various allergic symptomatologies - including dermatographia, rhinitis, conjunctivitis, and urticaria - as well as adjunctive therapy in the management of anaphylaxis following treatment with epinephrine. In Canada, cyproheptadine is available over-the-counter and is indicated for the treatment of pruritus and for appetite stimulation. In Australia, cyproheptadine is additionally indicated for the treatment vascular headaches.
Cyproheptadine is also used off-label for the treatment of serotonin syndrome.
Background
Cyproheptadine is a potent competitive antagonist of both serotonin and histamine receptors. It is used primarily to treat allergic symptoms, though it is perhaps more notable for its use in appetite stimulation and its off-label use in the treatment of serotonin syndrome.
Definition
ChEBI: The product resulting from the formal oxidative coupling of position 5 of 5H-dibenzo[a,d]cycloheptene with position 4 of 1-methylpiperidine resulting in the formation of a double bond between the two fragments. t is a sedating antihistamine with antimuscarinic and calcium-channel blocking actions. It is used (particularly as the hydrochloride sesquihydrate) for the relief of allergic conditions including rhinitis, conjunctivitis due to inhalant allergens and food , urticaria and angioedema, and in pruritic skin disorders. Unlike other antihistamines, it is also a seratonin receptor antagonist, making it useful in conditions such as vascular headache and anorexia.
Manufacturing Process
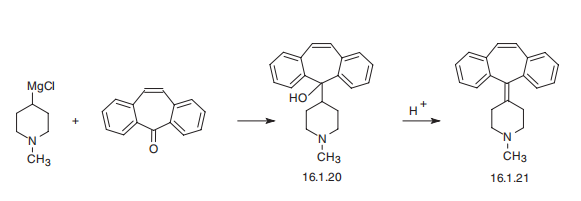
(A) Preparation of 1-Methyl-4-Piperidyl-Magnesium Chloride: Magnesium
turnings (5.45 g, 0.22 g-atom) were placed in a 500 ml 3-necked flask
provided with a condenser, Hershberg stirrer and dropping funnel and
protected with a drying tube. An atmosphere of dry nitrogen was maintained
in the apparatus throughout the reaction. The magnesium was covered with 20 ml of dry tetrahydrofuran. A crystal of iodine and 1.2 g of ethyl bromide
were added and after the reaction had subsided (formation of ethylmagnesium
bromide) a solution of 29.4 g (0.22 mol) of 4-chloro-1-methyl-piperidine in
dry tetrahydrofuran (total volume, 103 ml) was added dropwise at such a rate
that gentle reflux was maintained.
The solution of 4-chloro-1-methylpiperidine in tetrahydrofuran was dried over
calcium hydride at ice-bath temperature prior to use. When the addition of the
halide was complete the reaction mixture was refluxed with stirring for one
hour. In some subsequent experiments this period of refluxing was omitted
with no deleterious result.
The solution of 4-chloro-1-methylpiperidine in tetrahydrofuran was dried over
calcium hydride at ice-bath temperature prior to use. When the addition of the
halide was complete the reaction mixture was refluxed with stirring for one
hour. In some subsequent experiments this period of refluxing was omitted
with no deleterious result.
The solvent was evaporated from the combined benzene extracts to give 33.4
g of a clear light brown resin. Crystallization from an alcohol-water mixture
gave 19.5 g of 1-methyl-4-(5-hydroxy-5-dibenzo[a,e]cycloheptatrienyl)-
piperidine, MP 156° to 157°C. Two recrystallizations from alcohol-water
mixtures followed by two recrystallizations from benzene-hexane mixtures
gave analytically pure product, MP 166.7° to 167.7°C.
(C) Preparation of 1-Methyl-4-(5-Dibenzo[a,e]Cycloheptatrienylidene)-
Piperidine Hydrochloride: 1-Methyl-4-(5-hydroxy-5-dibenzo[a,e]
cycloheptatrienyl)-piperidine (3.05 g, 0.01 mol) was dissolved in glacial acetic
acid, 15 ml. The solution was saturated with dry hydrogen chloride with
external cooling. A white solid separated. Acetic anhydride (3.07 g, 0.03 mol)
was added and the mixture heated on the steam bath for one hour. The solid
dissolved in the first 5 minutes of the heating period.
The reaction mixture was poured into 25 ml of water and the mixture made
strongly basic with 10N sodium hydroxide solution. The mixture was extracted
3 times with 50 ml portions of benzene, the combined extracts washed with
water and concentrated to a volume of approximately 50 ml. The solution was
saturated with dry hydrogen chloride and the white crystalline product
collected and dried. The yield of product, MP 251.6° to 252.6°C (dec.) was
2.5 g. Recrystallization from a mixture of absolute alcohol and absolute ether
gave a product, MP 252.6° to 253.6°C. A sample was analyzed after drying
for 7 hours at 110°C over phosphorus pentoxide in vacuo.
(D) Preparation of 1-Methyl-4-(5-Dibenzo[a,e]Cycloheptatrienylidene)-
Piperidine: The hydrochloride salt, 4.3 g, was suspended in 100 ml of warm
water and the mixture made strongly alkaline by the addition of 15 ml of 5%
sodium hydroxide. The mixture was extracted with four 50 ml portions of
benzene and the extracts dried over sodium sulfate. Evaporation of the
benzene on the steam-bath at reduced pressure left 3.7 g (97%) of the base,MP 110.3° to 111.3°C. Recrystallization from a mixture of alcohol and water
gave product, MP 112.3° to 113.3°C.
brand name
Periactin (Merck);Anarexal;Antegan;Apeplus;Brantina;Brantine;Carnigol;Carpantin;Ciplactin;Cipractin;Cipro n;Ciprocort;Cyrasarl;Estialim;Histatets;Ifrasarl;Kontrast u;Naidoretico;Nurdelin;Nuttriben;Oractine;Orexigen;Periactol;Perideca;Pranzo;Reparal carnitina;Siglatan;Sigloton;Sipraktin;Siprodin;Vimicon.
Therapeutic Function
Antipruritic, Antihistaminic, Appetite stimulant
World Health Organization (WHO)
Cyproheptadine, an antihistamine with anticholinergic and serotonin-antagonist properties, was introduced in 1961 for the symptomatic relief of allergy and was subsequently used as an appetite stimulant. In 1982 the drug was prohibited in Bangladesh because of its misuse as an appetite stimulant due to inappropriate promotion. Cyproheptadine remains widely available and the current marketing policy of the major manufacturer requires that it should be used as an appetite stimulant only under the supervision of a physician who should be assured that adequate food is available.
Pharmacokinetics
Cyproheptadine has been observed to antagonize several pharmacodynamic effects of serotonin in laboratory animals, including bronchoconstriction and vasodepression, and has demonstrated similar efficacy in antagonizing histamine-mediated effects. The reason for its efficacy in preventing anaphylactic shock has not been elucidated, but appears to be related to its anti-serotonergic effects.
Safety Profile
Poison by ingestion, intraperitoneal, subcutaneous and intravenous routes. Experimental teratogenic and reproductive effects. When heated to decomposition it emits toxic fumes of NOx.
Synthesis
Cyproheptadine, 4-(dibenzo[a,d]cyclohepten-5-ylidene)-1-methylpiperidine (16.1.21), is synthesized by reacting 1-methyl-4-magnesiumchloropiperidine with 5H-dibeno[a,d]cycloheptene-5-one, which forms carbinol (16.1.20), the dehydration of which in an acidic medium leads to the formation of cyproheptadine (16.1.21).
Metabolism
The principal metabolite found in human urine has been identified as a quaternary ammonium glucuronide conjugate of cyproheptadine.
Properties of Cyproheptadine
| Melting point: | 112.3-113.3° |
| Boiling point: | 419.7°C (rough estimate) |
| Density | 0.9917 (rough estimate) |
| refractive index | 1.8240 (estimate) |
| storage temp. | Store at -20°C |
| form | Solid |
| pka | pKa 8.87 (Uncertain) |
| color | Crystals from EtOH (aq) |
| Water Solubility | 317.6ug/L(22.5 ºC) |
| CAS DataBase Reference | 129-03-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Cyproheptadine(129-03-3) |
Safety information for Cyproheptadine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cyproheptadine
Cyproheptadine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Cyproheptadine 98%View Details
Cyproheptadine 98%View Details -
 Cyproheptadine 98%View Details
Cyproheptadine 98%View Details
129-03-3 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
