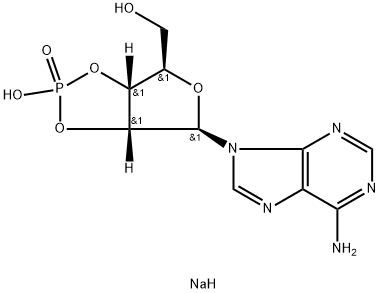
Cyclic AMP
- CAS NO.:60-92-4
- Empirical Formula: C10H12N5O6P
- Molecular Weight: 329.21
- MDL number: MFCD00005845
- EINECS: 200-492-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-21 21:32:25
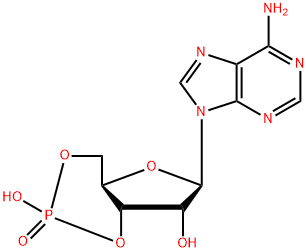
What is Cyclic AMP?
Chemical properties
White crystalline or Pale yellow powder; odorless. Slightly soluble in water, almost insoluble in ethanol or ether.
The Uses of Cyclic AMP
cAMP is used for intracellular signal transduction in many different organisms, conveying the cAMP-dependent pathway. Restoration of several morphological characteristics of normal fibroblasts in sarcoma cells when treated with adenosine-3': 5'-cyclic monophosphate. In the presence of adenosine 3':5'-cyclic monophosphate, protein kinase I dissociated into two subunits: a subunit binding adenosine 3':5'-cyclic monophosphate, and a catalytic subunit. Stimulation of calcium uptake in platelet membrane vesicles by adenosine 3',5'-cyclic monophosphate.
What are the applications of Application
Adenosine 3′,5′-cyclic monophosphate is a second messenger and key intracellular regulator
Definition
ChEBI: Cyclic AMP is a 3',5'-cyclic purine nucleotide having having adenine as the nucleobase. It has a role as a human metabolite, an Escherichia coli metabolite and a mouse metabolite. It is an adenyl ribonucleotide and a 3',5'-cyclic purine nucleotide. It is a conjugate acid of a 3',5'-cyclic AMP(1-).
General Description
Cyclic AMP is a second messenger molecule comprised of an adenine ribonucleotide bearing a phosphate group bound to the oxygen molecules at the 3' and 5' positions of the sugar moiety. Cyclic AMP, which is synthesized from ATP by the intracellular enzyme adenylate cyclase, modulates the activity of several hormone-dependent signal transduction pathways.
Biochem/physiol Actions
Naturally-occurring activator of cyclic-AMP-dependent protein kinase (PKA). cAMP is an important second messenger that is linked in many systems to neurotransmitter- or hormone-induced receptor stimulation. The cAMP/PKA signaling pathway has been shown to inhibit cell proliferation, induce differentiation and lead to apoptosis.
Safety Profile
Human mutation data reported.When heated to decomposition it emits toxic fumes ofPOx and NOx.
Synthesis
Cyclic AMP is synthesized from ATP by adenylate cyclase located on the inner side of the plasma membrane and anchored at various locations in the interior of the cell.[1] Adenylate cyclase is activated by a range of signaling molecules through the activation of adenylate cyclase stimulatory G (Gs)-protein-coupled receptors. Adenylate cyclase is inhibited by agonists of adenylate cyclase inhibitory G (Gi)-protein-coupled receptors. Liver adenylate cyclase responds more strongly to glucagon, and muscle adenylate cyclase responds more strongly to adrenaline.
cAMP decomposition into AMP is catalyzed by the enzyme phosphodiesterase.
Properties of Cyclic AMP
| Melting point: | 260 °C (dec.)(lit.) |
| Boiling point: | 56.12°C |
| alpha | -53.7 º (c=0.7,water) |
| Density | 2.47±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | H2O: 10 mg/mL pH of aqueous solution is approx. 3.0. The sodium salt (A6885) is about 20× more soluble., clear, colorless |
| pka | 1.00±0.60(Predicted) |
| form | powder |
| color | white |
| Water Solubility | 50 mg/mL |
| Merck | 14,2708 |
| BRN | 52645 |
| Stability: | Hygroscopic |
| NIST Chemistry Reference | Adenosine 3',5'-cyclic monophosphate(60-92-4) |
| EPA Substance Registry System | Adenosine, cyclic 3',5'-(hydrogen phosphate) (60-92-4) |
Safety information for Cyclic AMP
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P363:Wash contaminated clothing before reuse. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
Computed Descriptors for Cyclic AMP
Related products of tetrahydrofuran
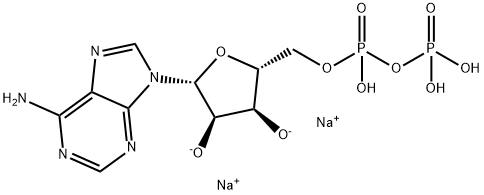
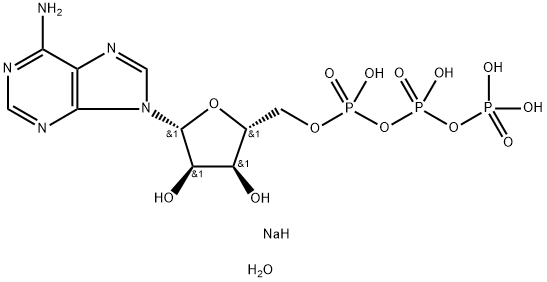
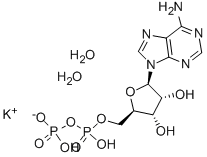
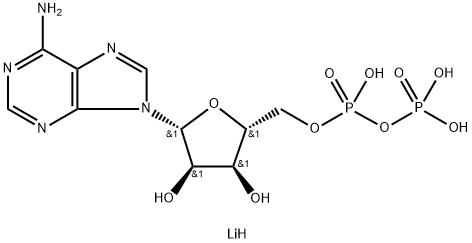
You may like
-
 Adenosine 3',5'-Cyclic Monophosphate CAS 60-92-4View Details
Adenosine 3',5'-Cyclic Monophosphate CAS 60-92-4View Details
60-92-4 -
 Cyclic AMP 98% CAS 60-92-4View Details
Cyclic AMP 98% CAS 60-92-4View Details
60-92-4 -
 Adenosine-3’,5’-Cyclic-Monophosphoric Acid extrapure CAS 60-92-4View Details
Adenosine-3’,5’-Cyclic-Monophosphoric Acid extrapure CAS 60-92-4View Details
60-92-4 -
 Adenosine 3′,5′-cyclic monophosphate, ≥98.5% (HPLC), powder CAS 60-92-4View Details
Adenosine 3′,5′-cyclic monophosphate, ≥98.5% (HPLC), powder CAS 60-92-4View Details
60-92-4 -
 Adenosine 3′,5′-cyclic monophosphate CAS 60-92-4View Details
Adenosine 3′,5′-cyclic monophosphate CAS 60-92-4View Details
60-92-4 -
 37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 -
 104944-18-5 99%View Details
104944-18-5 99%View Details
104944-18-5 -
 3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
37951-49-8