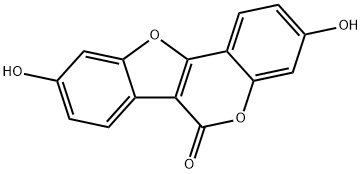
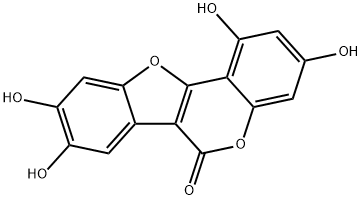
COUMESTROL
Synonym(s):7,12-Dihydroxycoumestan
- CAS NO.:479-13-0
- Empirical Formula: C15H8O5
- Molecular Weight: 268.22
- MDL number: MFCD00016885
- EINECS: 207-525-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
What is COUMESTROL?
Description
Coumestrol is a phytoestrogen that occurs naturally in soybeans, spinach, and clover. It competitively (vs. 17β-
Chemical properties
Yellow to Beige Powder
The Uses of COUMESTROL
This compound has estrogenic activity. It exhibits bright blue fluorescence in neutral or acid solution, and greenish-yellow fluorescence in strong alkali solution.
The Uses of COUMESTROL
This compound has estrogenic activity. It exhibits bright blue fluorescence in neutral or acid solution, and greenish-yellow fluorescence in strong alkali solution
Definition
ChEBI: A member of the class of coumestans that is coumestan with hydroxy substituents at positions 3 and 9.
What are the applications of Application
Coumestrol is a plant estrogen & highly potent inhibitor of 17-β-hydroxysteroid oxidoreductase
Synthesis Reference(s)
Tetrahedron Letters, 4, p. 1151, 1963 DOI: 10.1039/jr9630005127
Biochem/physiol Actions
Coumestrol has anti-estrogenic actions in the brain and pituitary, mediated through estrogen receptor-α.
Safety Profile
An experimental teratogen. Other experimental reproductive effects. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.
References
[1]. chen hy, dykstra kd, birzin et, et al. estrogen receptor ligands. part 1: the discovery of flavanoids with subtype selectivity. bioorg med chem lett. 2004 mar 22;14(6):1417-21.
[2]. hopert ac, beyer a, frank k, et al. characterization of estrogenicity of phytoestrogens in an endometrial-derived experimental model. environ health perspect. 1998 sep;106(9):581-6.
[3]. wang h, li h, moore lb, et al. the phytoestrogen coumestrol is a naturally occurring antagonist of the human pregnane x receptor. mol endocrinol. 2008 apr;22(4):838-57.
Properties of COUMESTROL
| Melting point: | ≥350 °C(lit.) |
| Boiling point: | 331.39°C (rough estimate) |
| Density | 1.2586 (rough estimate) |
| refractive index | 1.7680 (estimate) |
| storage temp. | Refrigerator |
| solubility | DMSO: soluble |
| form | Light beige solid. |
| pka | 8.25±0.20(Predicted) |
| color | Pale Yellow to Dark Brown |
| BRN | 266702 |
| CAS DataBase Reference | 479-13-0(CAS DataBase Reference) |
| EPA Substance Registry System | Coumestrol (479-13-0) |
Safety information for COUMESTROL
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for COUMESTROL
COUMESTROL manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![1-[2-Hydroxy-4-methoxy-5-(3-methyl-2-butenyl)phenyl]-3-(4-hydroxyphenyl)-2-propen-1-one](https://img.chemicalbook.in/CAS/GIF/20784-60-5.gif)


You may like
-
 479-13-0 Coumestrol ( synthitic) 98%View Details
479-13-0 Coumestrol ( synthitic) 98%View Details
479-13-0 -
 479-13-0 98%View Details
479-13-0 98%View Details
479-13-0 -
 479-13-0 Coumestrol 98%View Details
479-13-0 Coumestrol 98%View Details
479-13-0 -
 Coumestrol 95% CAS 479-13-0View Details
Coumestrol 95% CAS 479-13-0View Details
479-13-0 -
 Coumestrol CAS 479-13-0View Details
Coumestrol CAS 479-13-0View Details
479-13-0 -
 Coumestrol CAS 479-13-0View Details
Coumestrol CAS 479-13-0View Details
479-13-0 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1