Corticotropin
- CAS NO.:9002-60-2
- Empirical Formula: C207H308N56O58S
- Molecular Weight: 4541.1
- MDL number: MFCD00167432
- EINECS: 232-659-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-12 17:22:58
What is Corticotropin?
Description
Natural corticotropin is a 39-amino-acid polypeptide secreted by the anterior pituitary gland; it is obtained from animal pituitaries. The physiological activity resides in the first 24 amino acids (which are common to many species) and most immunological activity lies in the remaining 15 amino acids. The pituitary output of corticotropin responds rapidly to physiological requirements by the familiar negativefeedback homeostatic mechanism. As the t½ of corticotropin is 10 min and the adrenal cortex responds within 2 min, corticosteroid output can adjust rapidly.
Synthetic corticotropins have the advantage of shorter amino acid chains (they lack amino acids 25–39) which are less likely to cause serious allergy, although this does occur. Additionally, they are devoid of animal proteins, which are potent allergens. Tetracosactide (tetracosactrin) consists of the biologically active first 24 amino acids of natural corticotropin (from humans or animals) and so it has similar properties, e.g. t½ 10 min.
The Uses of Corticotropin
Hormone (adrenocorticotropic); glucocorticoid; diagnostic aid (adrenocortical insufficiency) Acthar (Sanofi Aventis); Cortrophin (Organon) [Name previously used: Corticotrophin].
Definition
ChEBI: A polypeptide hormone produced and secreted by the pituitary gland comprising 39 amino acid residues coupled in a linear sequence. The N-terminal 24-amino acid segment is identical in all species and contains the adrenocorticotrophic act vity. Corticotropin stimulates the cortex of the adrenal gland and boosts the synthesis of corticosteroids, mainly glucocorticoids but also sex steroids (androgens). It is used in the treatment of certain neurological disorders such as infantile spasms and multiple sclerosis, and diagnostically to investigate adrenocortical insufficiency.
Indications
Corticotropin (ACTH, Acthar, Cortrophin Gel) is an open-chain polypeptide that consists of 39 amino acid residues, the first 24 of which are essential for its biological activity. The remainder of the amino acids are also clinically important, since they may be involved in stimulating antibody formation and causing allergic reactions. This is true especially when corticotropin of animal origin is injected into humans. Commercially available corticotropin is prepared from animal pituitary glands.
brand name
Cortigel (Savage); H.P. Acthar Gel (Rhone-Poulenc Rorer).
Biological Activity
ACTH is the adrenocorticotropic hormone of the anterior lobe of the pituitary gland, which specifically stimulates the adrenal cortex to secrete cortisone, and hence has effects identical with those of cortisone. ACTH differs in its chemistry, absorption, and metabolism from the other adrenal steroids. Chemically, it is a watersoluble polypeptide having a molecular weight of about 3500. Its complete amino acid sequence has been determined. It produces its peripheral physiological effects by causing discharge of the adrenocortical steroids into the circulation. ACTH has been extracted from pituitary glands. In purified form, ACTH is useful in treating some forms of arthritis, lupus erythematosus, and severe skin disorders. The action of ACTH injections parallels the result of large quantities of naturally formed cortisone if they were released naturally.
Mechanism of action
Corticotropin stimulates the synthesis of corticosteroids (of which the most important is cortisol) and to a lesser extent of androgens, by the cells of the adrenal cortex. It has only a minor (transient) effect on aldosterone production, which proceeds independently; in the absence of corticotropin the cells of the inner cortex atrophy.
The release of natural corticotropin by the pituitary gland is controlled by the hypothalamus through corticotropin releasing hormone (CRH, or corticoliberin), production of which is influenced by environmental stresses as well as by the level of circulating cortisol. High plasma concentration of any adrenal steroid with glucocorticoid effect prevents release of CRH and so of corticotropin, lack of which in turn results in adrenocortical hypofunction. This is why catastrophe may accompany abrupt withdrawal of long-term adrenal steroid therapy with adrenal atrophy.
The effects of corticotropin are those of the steroids (hydrocortisone, androgens) liberated by its action on the adrenal cortex. Prolonged heavy dosage causes the clinical picture of Cushing's syndrome.
Pharmacology
Corticotropin is rapidly inactivated by gastrointestinal proteolytic enzymes and therefore must be administered parenterally. It is rapidly removed from the circulation (T1/2, 15 minutes) and is probably inactivated in body tissues, since no intact compound is found in the urine.
Clinical Use
Corticotropin is used principally in diagnosis and rarely in treatment. It is inactive if taken orally and has to be injected like other peptide hormones.
Diagnostic use is to test the capacity of the adrenal cortex to produce cortisol. With the short synacthen test, the plasma cortisol concentration is measured before and 30 min and 60 min after an intramuscular injection of 250 micrograms of tetracosactide (Synacthen); a normal response is a rise in plasma cortisol concentration of more than 200 nmol/L or a peak of greater than 500 nmol/L at 30 or 60 min. In cases of uncertainty, the longer variants of the test require intramuscular injection of a depot (sustained-release) formulation, e.g. 1 mg daily for 3 days at 09:00 hours, with a short tetracosactide test performed on day 3.
Therapeutic use is seldom appropriate, as the peptide hormone must be injected. Selective glucocorticoid (without mineralocorticoid) action is not possible, and clinical results are irregular. Corticotropin cannot be relied on to restore adrenal cortisol output when a steroid is being withdrawn after prolonged therapy, as it does not restore function in the suppressed hypothalamic–pituitary part of the HPA axis.
Clinical Use
The rationale for using corticotropin instead of pharmacological concentrations of glucocorticoids stems from the fact that corticotropin provides enhanced amounts of all endogenously secreted adrenocortical hormones, including androgens. However, obvious disadvantages are associated with the use of this polypeptide: (1) It must be given daily parenterally. (2) It is quite expensive. (3) It is antigenic and thus can produce resistance and hypersensitivity reactions. Corticotropin is used as a diagnostic tool for the identification of primary adrenal insufficiency or as a method for evaluating the hypothalamic–pituitary–adrenal axis before surgery in patients previously treated with glucocorticoids.
Side Effects
Aside from hypersensitivity and allergic reactions, corticotropin administration has been associated with electrolyte disturbances and masculinization in women.
Veterinary Drugs and Treatments
Availability of corticotropin in FDA-approved products is an issue
as no commercially products were commercially available for veterinary
use at the time writing and either cosyntropin (see monograph)
or compounded ACTH products are required.
In veterinary medicine, an ACTH product (Adrenomone?—
Summit Hill) was approved
for use in dogs, cats, and beef or dairy
cattle for stimulation of the adrenal cortex when there is a deficiency
of ACTH and as a therapeutic agent in primary bovine ketosis,
but apparently is no longer commercially available. In practice,
ACTH tends to be used most often in the diagnosis of hyper- or
hypoadrenocorticism (ACTH-stimulation test) and to monitor the
response to mitotane therapy in Cushing’s syndrome.
One reference (Behrend 2003a) recommends using the ACTH
stimulation test if the dog has non-adrenal illness, received any
form of exogenous glucocorticoids (including topicals), or received
phenobarbital. If the dog has no known non-adrenal illness and
moderate to severe clinical signs of hyperadrenocorticism, use the
low-dose dexamethasone suppression test. If using the ACTH-stim
test, the author states that cosyntropin is the agent of choice (see
that monograph).
ACTH has been used for several purposes in human medicine
for its corticosteroid stimulating properties, but as it must be injected,
it is not commonly employed in veterinary patients.
Properties of Corticotropin
| storage temp. | −20°C |
| solubility | H2O: 1 mg/mL, clear, colorless |
| form | powder |
| Merck | 13,136 |
| CAS DataBase Reference | 9002-60-2 |
Safety information for Corticotropin
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H332:Acute toxicity,inhalation H334:Sensitisation, respiratory H361:Reproductive toxicity |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P342+P311:IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. |
Computed Descriptors for Corticotropin
New Products
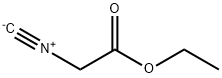
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1