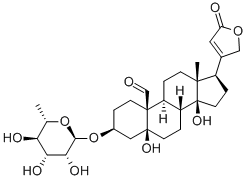
CONVALLATOXIN
Synonym(s):3β,5α,14-Trihydroxy-19-oxo-5β,20(22)-cardenolide 3-(6-deoxy-α-L -mannopyranoside);Strophanthidin α-L -rhamnopyranoside
- CAS NO.:508-75-8
- Empirical Formula: C29H42O10
- Molecular Weight: 550.64
- MDL number: MFCD00069477
- EINECS: 208-086-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
What is CONVALLATOXIN?
Originator
Convallatoxin,C-Strong Co., Ltd.
The Uses of CONVALLATOXIN
Convallatoxin is a cardenolide that is used in the treatment of congestive heart failure. It may also be used in the treatment of tumor cells at nanomolar concentrations due to its anti-proliferative effects
Definition
ChEBI: A cardenolide glycoside that consists of strophanthidin having a 6-deoxy-alpha-L-mannopyranosyl (L-rhamnosyl) group attached at position 3.
Manufacturing Process
1 part of grinded flowers Convallaria majalis and 12 parts of water was stirred
for 15 hours at ambient temperature. After a filtration and washing with
water, a clear brown filtrate was mixed with a concentrate solution of lead
acetate. A lead consisted precipitate was filtered off and sodium phosphate
was added to filtrate for removing the remaining lead. The solution was
filtered again and 0.5 - 0.6 parts of a coal was added and the mixture was
stirred for 3 hours at ambient temperature. The coal was filtered off, washed
with a little water and dried at 30°-40°C. A hot CHCl3 was added to dry coal
adsorbent. CHCl3 was distilled off to dryness in vacuum. The residue was
dissolved in a little methanol and the obtained solution was shook 3 times
with 2 volumes of petrol ether and then distilled to dryness in vacuum. This
product was dissolved in minimum absolute ethanol and added to 10 volumes
of dry ether. The formed precipitate was filtered and washed with ether to
give the glycoside as a gray powder. It was crystallized from diluted ethanol
as colorless needles.
Therapeutic Function
Cardiotonic
Purification Methods
Crystallise convallatoxin from EtOAc, CHCl3/EtOH (9:1) or MeOH/Et2O. The tetraacetate has m 238-242o (from MeOH/Et2O), [] D 25 -5o (CHCl3). [Reyle et al. Helv Chim Acta 33 1541 1950, Fieser & Jacobson J Am Chem Soc 59 2335 1937 Beilstein 1 8 III/IV 3142.]
Properties of CONVALLATOXIN
| Melting point: | 238-239℃ (water ) |
| Boiling point: | 542°C (rough estimate) |
| alpha | D22 -1.7 ± 3° (c = 0.65 in methanol); D25 -9.4 ± 3° (c = 0.72 in dioxane) |
| Density | 1.41±0.1 g/cm3 (20 ºC 760 Torr) |
| refractive index | 1.5376 (estimate) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| pka | 13.04±0.70(Predicted) |
| form | Solid |
| color | White to Off-White |
| Merck | 13,2538 |
| Stability: | Hygroscopic |
Safety information for CONVALLATOXIN
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for CONVALLATOXIN
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
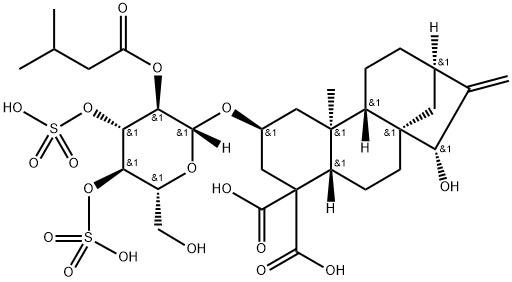
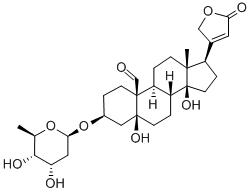
![dipotassium dihydrogen 15alpha-hydroxy-2beta-[[2-O-isovaleryl-3,4-di-O-sulphonato-beta-D-glucopyranosyl]oxy]kaur-16-ene-18,19-dioate](https://img.chemicalbook.in/CAS/GIF/33286-30-5.gif)
You may like
-
 Convallatoxin CAS 508-75-8View Details
Convallatoxin CAS 508-75-8View Details
508-75-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8