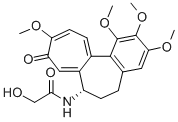
Colchicine
Synonym(s):(S)-N-(5,6,7,9-Tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[a]heptalen-7-yl)acetamide;Colchicine;Colchicine, Colchicum autumnale - CAS 64-86-8 - Calbiochem
- CAS NO.:64-86-8
- Empirical Formula: C22H25NO6
- Molecular Weight: 399.44
- MDL number: MFCD00078484
- EINECS: 200-598-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
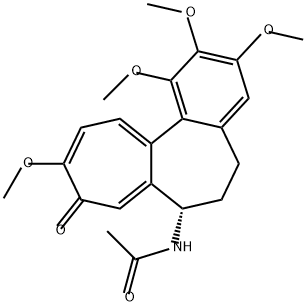
What is Colchicine?
Absorption
Colchicine is rapidly absorbed after oral administration from the gastrointestinal tract. The bioavailability of colchicine is about 45%: one study suggests that colchicine bioavailability is highly variable, ranging from 24 to 88%.
In healthy adults, the mean Cmax of 2.5 ng/mL (range 1.1 to 4.4 ng/mL) was achieved in one to two hours (range 0.5 to 3 hours) after a single dose administered under fasting conditions. In a multiple-dose study of colchicine administration at a dose of 1 mg per day, steady-state concentrations were achieved by day 8 following administration.
Administration of colchicine with food does not affect the colchicine absorption rate but decreases the extent of colchicine by approximately 15%.
Toxicity
The oral LD50 of colchicine in mice is 5.87 mg/kg.
Acute overdose exceeding 0.5 mg/kg (35 mg for a 70 kg average adult) is usually fatal. Fatalities have been reported with as little as 7 mg. Colchicine poisoning presents in three sequential and usually overlapping phases. The first stage of acute colchicine toxicity typically occurs within 24 hours of ingestion and includes gastrointestinal symptoms such as abdominal pain, nausea, vomiting, diarrhea, and significant fluid loss, leading to volume depletion. Peripheral leukocytosis may also be seen. The second stage develops 24 to 72 hours after ingestion and is characterized by multi-organ failure. Death is usually a result of respiratory depression and cardiovascular collapse. Recovery may be accompanied by rebound leukocytosis about one week after ingestion.
No specific antidote is known. Elimination of toxins by gastric lavage followed by activated charcoal should be attempted within 1-2 hours of ingestion. Colchicine is not effectively removed by hemodialysis.
Description
Colchicine is a pale-yellow powder that is obtained from various species of Colchicum, primarily Colchicum autumnale L. Its total chemical synthesis has been achieved, but the primary source of colchicine currently remains alcohol extraction of the alkaloid from the corm and seed of C. autumnale L. It darkens on exposure to light and possesses
Description
Robert Burns Woodward is generally considered to be the greatest synthetic organic chemist of the 20th century. From the 1940s to the 1970s, he led the syntheses of numerous natural products and pharmaceuticals. In 1965, Woodward received the Nobel Prize in Chemistry for this body of work.
Over the years, Molecule of the Week has featured several “Woodward molecules”, including?quinine,?cholesterol,?cortisone,?strychnine, and?chlorophyll. Here are four more of those important compounds with the dates their syntheses were reported.
Reserpine?(1958) was originally isolated from?Rauwolfia serpentina?in 1952. It was once used as a treatment for high blood pressure and psychotic episodes, but it has been replaced by newer drugs with fewer side effects.
Lysergic acid?(1956) is the biosynthetic precursor for several ergoline alkaloids. Its most notorious end product is LSD (lysergic acid diethylamide), a psychedelic drug promoted by Timothy Leary in the 1960s.
Cephalosporin C?(1966) is a member of the cephalosporin family of β-lactam antibiotics. They were discovered in 1945 in?Acremonium?fungus species.
Colchicine?(1963), primarily used to treat gout, dates to prehistoric times when its source, the?Colchicum autumnale?(crocus) plant, was used to treat rheumatism and swelling.
Woodward’s crowning synthetic accomplishment was vitamin B12?(cobalamin) in 1972. For this work, he collaborated with Swiss chemist Albert Eschenmoser and more than 100 graduate students and postdoctoral fellows. Each of the research groups contributed a portion of the molecule.
Woodward’s achievements in physical organic chemistry were also ground-breaking. Using molecular orbital theory, he and theoretical chemist Roald Hoffmann formulated rules that govern pericyclic reactions, such as cycloadditions. Hoffmann was awarded the 1981 Nobel Prize in chemistry for the Woodward–Hoffman rules, 2 years after Woodward’s death.
Bethany Halford’s article in this week’s issue of C&EN?commemorates Woodward’s career.
Chemical properties
Yellow Solid
Chemical properties
Colchicine is a pale yellow powder. It has little or no odor. It darkens on contact with light.
Physical properties
Appearance: colchicine exists in white or light-yellow crystal powder with no smell, and it is seldom prone to absorb moisture. Melting point: it becomes dark when it is exposed to light, and it melts at 87–89?°C. Solubility: this product is soluble in chloroform or ethanol and it dissolves in water. However, the semihydrate crystal can form in certain concentrations. The product is hardly soluble in ether. Specific optical rotation: ?121° (0.9?g/100?mL, chloroform, 589.3?nm, 17?°C).
History
Meadow saffron (Colchicum) is recorded to treat rheumatic swelling on ancient
Egyptian medical papyrus in 1500 B.C.. According to De Materia Medica written by Pedanius Dioscorides in the first century, extract of Meadow saffron is used
in treating gout. London Pharmacopoeia in 1618 recorded that colchicine is also
applied to treat gout.
In 1820, the ingredient was first isolated by the French chemist P.S. Pelletier and
J.B. Caventou. In 1833, it was purified and named by Geiger. Michael Dewar
guessed that there are two seven-membered rings in colchicine in 1945. Murray
Vernon King et al. determined the structure of colchicine by X-ray diffraction in
1952. In 1959, Albert Eschenmoser integrated the product successfully
Colchicine tablet and raw material are approved mostly in domestic in 2010. The
tablet produced by Taiwan manufacturers is approved for being listed in mainland
of China in 2012. The raw material made by Indian obtained the approval in 2013.
There are three kinds of colchicine approved by FDA: with the combination of probenecid, it is prior to be approved. The others are tablet (2009) and capsule (2014).
The Uses of Colchicine
Colchicine is present in the poisonous autumncrocus (meadow saffron). It is the major alkaloid of Colchicum autumnale L. and Liliaceae. It was used in poison potions in theancient kingdom of Colchis (Greece). It isused therapeutically as an antineoplast, for thesuppression of gout, and in the treatment ofMediterranean fever. It is used in plant studiesfor doubling chromosome groups.
The Uses of Colchicine
Binds specifically with tubulin thus interfering with microtubule organization.
The Uses of Colchicine
antimitotic, antigout agent
The Uses of Colchicine
An antimitotic agent that disrupts microtubles by binding to tubulin and preventing its polymerization. Stimulates the intrinsic GTPase activity of tubulin. Induces apoptosis in several normal and t umor cell lines and activates the JNK/SAPK signaling pathway.
The Uses of Colchicine
anti-inflammatory agent, mitosis inhibitor
The Uses of Colchicine
For treatment and relief of pain in attacks of acute gouty arthritis.
The Uses of Colchicine
In research in plant genetics (for doubling chromosomes).
Background
Colchicine is an alkaloid drug derived from a plant belonging to the Lily family, known as Colchicum autumnale, or "autumn crocus." Its use was first approved by the FDA in 1961. Colchicine is used in the treatment of gout flares and Familial Mediterranean fever, and prevention of major cardiovascular events. It has also been investigated in other inflammatory and fibrotic conditions.
Indications
Colchicine is indicated for the prophylaxis and treatment of gout flares. It is also indicated in Familial Mediterranean fever (FMF) in children and adults of four years of age and older. It is also indicated to reduce the risk of myocardial infarction (MI), stroke, coronary revascularization, and cardiovascular death in adult patients with established atherosclerotic disease or with multiple risk factors for cardiovascular disease.
Some off-label uses of colchicine include the treatment of the manifestations of Behcet's syndrome, pericarditis, and postpericardiotomy syndrome.
What are the applications of Application
Colchicine is a mitosis inhibitor useful in cell division studies
Definition
An alkaloid plant hormone.
Definition
colchicine: An alkaloid derivedfrom the autumn crocus, Colchicumautumnale. It inhibits cell division.Colchicine is used in genetics, cytology,and plant breeding research andalso in cancer therapy to inhibit celldivision.
Indications
Colchicine, an alkaloid obtained from the autumn crocus, has long been used and is relatively selective for the treatment of acute gouty arthritis. Unlike many of the newer agents for use in gout, colchicine has minimal effects on uric acid synthesis and excretion; it decreases inflammation associated with this disorder. It is thought that colchicine somehow prevents the release of the chemotactic factors and/or inflammatory cytokines from the neutrophils, and this in turn decreases the attraction of more neutrophils into the affected area .The ability of colchicine to bind to leukocyte microtubules in a reversible covalent complex and cause their depolymerization also may be a factor in decreasing the attraction of the motile leukocytes into the inflamed area.
Biological Functions
Acting on polymorphonuclear leukocytes and diminishing phagocytosis, it inhibits the production of lactic acid, causing an increase in the pH of synovial tissue and, thus, a decrease in urate deposition, because uric acid is more soluble at the higher pH. Additionally, colchicine inhibits the release of lysosomal enzymes during phagocytosis that also contributes to the reduction of inflammation. Because colchicine does not lower serum urate levels, it has been found to be beneficial to combine colchicine with a uricosuric agent, particularly probenecid. It is a potent drug, being effective at doses of approximately 1 mg, but doses as small as 7 mg have caused fatalities.
General Description
Odorless or nearly odorless pale yellow needles or powder that darkens on exposure to light. Used to treat gouty arthritis, pseudogout, sarcoidal arthritis and calcific tendinitis.
General Description
Colchicine is an alkaloid isolated from the dried corns andseeds of Colchicum autumnale L., commonly known as autumncrocus or meadow saffron.It is specifically indicated for acute treatment of goutyarthritis because of its ability to block the production and releaseof the CCF that mediates the inflammatory responsebecause of urate crystals, a mechanism different fromcolchicine’s antimitotic action, which is being investigatedfor its anticancer properties. It is often quite effective inaborting an acute gouty attack if given within the first 10 to12 hours after the onset of arthritis.
Air & Water Reactions
Slowly hydrolyzed in acidic solution, but unbuffered solutions are stable at 68°F for at least six months. Isomerizes on exposure to ultraviolet radiation.
Reactivity Profile
Colchicine darkens on exposure to light. Incompatible with strong oxidizing agents. Also incompatible with mineral acids .
Hazard
As little as 20 mg may be fatal if ingested.
Health Hazard
Colchicine is classified as super toxic. Probable oral lethal dose in humans is less than 5 mg/kg, i.e. less than 7 drops for a 70 kg (150 lb.) person. Death results from respiratory arrest. The fatal dose varies considerably; as little as 7 mg of Colchicine has proved fatal.
Health Hazard
Colchicine is a highly toxic alkaloid. Thetarget organs are the lungs, kidney, gastrointestinal tract, and blood. The toxiceffects include drowsiness, nausea, hypermotility, diarrhea, lowering of body temperature, lowering of blood pressure, tremors,convulsions, and respiratory distress. Chronicingestion may cause aplastic anemia andhemorrhage.
LD50 value, oral (mice): 5.9 mg/kg
Colchicine solution at 10,000 ppm concentration caused severe irritation whenapplied repeatedly to rabbits’ eyes. Colchicine produced teratogenic effects in testanimals. It caused fetal death, cytologicalchanges, and developmental abnormalitiesin hamsters, rabbits, domestic animals,and mice. It tested positive to in vitromammalian nonhuman micronucleus andD. melanogaster — nondisjunction tests formutagenicity (U.S. EPA 1986; NIOSH1986).
Fire Hazard
Stable.
Biological Activity
Plant-derived alkaloid that binds to tubulin and depolymerizes microtubules.
Biochem/physiol Actions
Colchicine interacts with albumin and binds to tubulin. Its association with tubulin impacts autophagic vacuole fusion with lysosomes. It inhibits tyrosine kinases and phospholipases. Colchicine may be useful for treating acute coronary syndromes. It is prescribed for treating rheumatologic conditions including familial mediterranean fever (FMF) and acute gouty arthritis.
Mechanism of action
Colchicine is rapidly absorbed after oral administration and tends to concentrate in the spleen, kidney, liver, and gastrointestinal tract. Leukocytes also avidly accumulate and store colchicine even after a single intravenous injection. Since colchicine can accumulate in cells against a concentration gradient, it is postulated that an active transport process may be involved in its cellular uptake. The drug is metabolized, primarily in the liver, by deacetylation. Fecal excretion plays a major role in colchicine elimination, since it and its metabolites are readily secreted into the bile. Only about 15 to 30% of the drug is eliminated in the urine except in patients with liver disease; urinary excretion is more important in these individuals.
Pharmacokinetics
Colchicine ameliorates the symptoms of gout and Familial Mediterranean fever. It possesses anti-inflammatory, anti-fibrotic, and cardiovascular protective effects. Colchicine was shown to exhibit anticancer properties, such as the inhibition of cancer cell migration and angiogenesis. Colchicine has a narrow therapeutic window.
Pharmacology
The drug can be given intravenously as well as orally, but care must be exercised, since extravasated drug may result in local sloughing of skin and subcutaneous tissues. Relief of pain and inflammation usually occurs within 48 hours. Small doses of colchicine can be used during asymptomatic periods to minimize the reappearance or severity of acute attacks. It should be used with caution in patients with preexisting compromised heart, kidney, gastrointestinal tract, and liver disease. Diarrhea, nausea, vomiting, and abdominal pain are the major limiting side effects that ultimately determine the tolerated dosage. These symptoms occur in approximately 80% of patients who take colchicine, especially in those taking high dosages. The hepatobiliary recycling of colchicine and its antimitotic effect on cells that are rapidly turning over, such as those of the intestinal epithelium, account for its gastrointestinal toxicity. Gastrointestinal symptoms generally intervene before the appearance of more serious toxicity and thereby provide a margin of safety in drug administration. Ingestion of large doses of colchicine may be followed by a burning sensation in the throat, bloody diarrhea, shock, hematuria, oliguria, and central nervous system (CNS) depression.
Pharmacokinetics
Colchicine is absorbed on oral administration, with peak plasma levels being attained within 0.5 to 2 hours after dosing. Plasma protein binding is only 31%. It concentrates primarily in the intestinal tract, liver, kidney, and spleen and is excreted primarily in the feces, with only 20% of an oral dose being excreted in the urine. It is retained in the body for considerable periods of time, being detected in the urine and leukocytes for 9 to 10 days following a single dose.
Anticancer Research
It is a natural toxic secondary metabolite, extracted from Colchicum genus plants. Itprevents gastric cancer by upregulating the dual specificity phosphatase 1 (DUSP1)gene. It is also reported to upregulate transforming growth factor beta 2 (TGF-β2)and A-kinase anchoring protein 12 (AKAP12) in hepatocellular carcinoma (Singhet al. 2016b).
Clinical Use
The major use of colchicine is as an antiinflammatory agent in the treatment of acute gouty arthritis; it is not effective in reducing inflammation in other disorders. It also can be used to prevent attacks. Since colchicine is so rapidly effective in relieving the acute symptoms of gout (substantial improvement is achieved within hours), it has been used as a diagnostic aid in this disorder. Therapy with colchicine is usually begun at the first sign of an attack and is continued until symptoms subside, adverse gastrointestinal reactions appear, or a maximum dose of 6 to 7 mg has been reached.
Side Effects
Colchicine may produce bone marrow depression, with long-term therapy resulting in thrombocytopenia or aplastic anemia. At maximum dose levels, GI disturbances (e.g., nausea, diarrhea, and abdominal pain) may occur. Acute toxicity is characterized by GI distress, including severe diarrhea resulting in excessive fluid loss, respiratory depression, and kidney damage. Treatment normally involves measures that prevent shock as well as morphine and atropine to diminish abdominal pain. A number of drug interactions have been reported. In general, the actions of colchicine are potentiated by alkalinizing substances and are inhibited by acidifying drugs, consistent with its mechanism of action of increasing the pH of synovial fluid. Responses to CNS depressants and to sympathomimetic drugs appear to be enhanced. Clinical tests may be affected; most notably, elevated alkaline phosphatase and SGOT (serum glutamate oxaloacetate transaminase) values and decreased thrombocyte values may be obtained.
Safety Profile
experimentally by most routes. Human systemic effects: aplastic anemia, blood pressure depression, body temperature decrease, changes in kidney tubules, dyspnea, flaccid paralysis without anesthesia, gastrointestinal effects, kidney damage and hemorrhaging, muscle contraction or spasticity, muscle weakness, nausea or vomiting, respiratory stimulation, and somnolence. An experimental teratogen. Experimental reproductive effects. A severe eye irritant. Human mutation data reported. Inhibits the formation of microtubules and thus impairs cell division. When heated to decomposition it emits toxic fumes of NOx.
Potential Exposure
Colchicine is a drug used to treat gouty arthritis, pseudogout, sarcoidal arthritis; and calcific tendonitis.
Veterinary Drugs and Treatments
In veterinary medicine, colchicine has been proposed as a treatment
in small animals for amyloidosis.
For colchicine to be effective,
however, it must be given early in the course of the disease and
it will be ineffective once renal failure has occurred. At the time of
writing, no conclusive evidence exists for its efficacy for this indication
in dogs.
Colchicine has also been proposed for treating chronic hepatic
fibrosis presumably by decreasing the formation and increasing the
breakdown of collagen.
Drug interactions
Potentially hazardous interactions with other drugs
Anti-arrhythmics: possible increased risk of toxicity
with amiodarone.
Antibacterials: possible increased risk of toxicity
with azithromycin, clarithromycin, erythromycin and
telithromycin - suspend or reduce dose of colchicine,
avoid concomitant use in renal or hepatic failure.
Antifungals: possible increased risk of toxicity with
itraconazole and ketoconazole - suspend or reduce
dose of colchicine, avoid concomitant use in renal or
hepatic failure.
Antivirals: possible increased risk of toxicity with
atazanavir, indinavir, ritonavir and telaprevir
- suspend or reduce dose of colchicine, avoid
concomitant use in renal or hepatic failure.
Calcium-channel blockers: possible increased risk of
toxicity with diltiazem and verapamil - suspend or
reduce dose of colchicine, avoid concomitant use in
renal or hepatic failure.
Cardiac glycosides: possible increased risk of
myopathy with digoxin.
Ciclosporin: risk of myopathy or rhabdomyolysis,
also increased blood-ciclosporin concentrations
and nephrotoxicity - suspend or reduce dose of
colchicine, avoid concomitant use in renal or hepatic
failure.
Grapefruit juice: possible increased risk of toxicity.
Lipid-regulating drugs: possible increased risk of
myopathy with fibrates and statins.
First aid
This material is an alkaloid. If this chemical getsinto the eyes, remove any contact lenses at once and irrigateimmediately for at least 15 min, occasionally lifting upper andlower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and washimmediately with soap and water. Seek medical attentionimmediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions,including resuscitation mask) if breathing has stopped andCPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Medicalobservation is recommended for 24-48 h after breathingoverexposure, as pulmonary edema may be delayed. As firstaid for pulmonary edema, a doctor or authorized paramedicmay consider administering a corticosteroid spray.
Environmental Fate
Colchicine binds to tubulin and prevents its polymerization into microtubules, subsequently disrupting microtubule function. Consequently, it alters nuclear structure, intracellular transport, and cytoplasmic motility, ultimately causing cell death. Colchicine is a potent inhibitor of cellular mitosis.
Metabolism
Colchicine is metabolized in the liver. It undergoes CYP3A4-mediated demethylation into major metabolites, 2-O-demethylcolchicine and 3-O-demethylcolchicine. It also forms one minor metabolite, 10-O-demethylcolchicine (colchiceine). Plasma levels of these metabolites are less than 5% of parent drug.
Metabolism
Metabolism occurs primarily in the liver, with the major metabolite being the amine resulting from amide hydrolysis.
Storage
Store at RT
Shipping
UN1544 Alkaloids, solid, n.o.s. or Alkaloid salts, solid, n.o.s. poisonous, Hazard Class: 6.1; Labels: 6.1- Poisonous materials, Technical Name Required. UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
Commercial material contains up to 4% desmethylcolchicine. Purify colchicine by chromatography on alumina and eluting with CHCl3 [Ashley & Harris J Chem Soc 677 1944]. Alternatively, an acetone solution on alkali-free alumina has been used, and eluting with acetone [Nicholls & Tarbell J Am Chem Soc 75 1104 1953]. It crystallises as yellow needles from EtOAc or CHCl3 with solvent of crystallisation which can be removed at ~70o. It is soluble in Et2O (0.5%), *C6H6 (1%) and H2O (4%). It is sold as “Colgout” for the treatment of gout and binds to tubulin. [Schreiber et al. Helv Chim Acta 44 540 1961, Scott et al. Tetrahedron 21 3605 1965, van Tamelen et al. Tetrahedron 14 8 1961, Beilstein 14 IV 946.]
Toxicity evaluation
No information is currently available on breakdown in soil,
groundwater, or surface water. Colchicine alkaloids withstand
storage, drying, and boiling.
Ingestion is the most common route of both accidental and
intentional exposure to colchicine. It is available as an oral
tablet and solution for injection.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, mineral acids. Keep away from light.
References
1) Merck 14:2471
Properties of Colchicine
| Melting point: | 150-160 °C (dec.)(lit.) |
| Boiling point: | 522.37°C (rough estimate) |
| alpha | -250 º (c=1, alcohol) |
| Density | 1.2770 (rough estimate) |
| refractive index | 1.5614 (estimate) |
| storage temp. | 2-8°C |
| solubility | H2O: 10 mg/mL |
| form | powder |
| pka | 12.36(at 20℃) |
| color | white to yellow with a green cast |
| Water Solubility | 45 g/L (20 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,2471 |
| BRN | 2228813 |
| Stability: | Stable. Light sensitive. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 64-86-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Colchicine(64-86-8) |
| EPA Substance Registry System | Colchicine (64-86-8) |
Safety information for Colchicine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H340:Germ cell mutagenicity |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. |
Computed Descriptors for Colchicine
| InChIKey | IAKHMKGGTNLKSZ-INIZCTEOSA-N |
Colchicine manufacturer
Kavya Pharma
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Colchicine 99%View Details
Colchicine 99%View Details -
 Colchicine 99%View Details
Colchicine 99%View Details -
 Colchicine ExiPlus CAS 64-86-8View Details
Colchicine ExiPlus CAS 64-86-8View Details
64-86-8 -
 Colchicine for tissue culture CAS 64-86-8View Details
Colchicine for tissue culture CAS 64-86-8View Details
64-86-8 -
 colchicine USP. CAS No:64-86-8, 1 Kg BagView Details
colchicine USP. CAS No:64-86-8, 1 Kg BagView Details
64-86-8 -
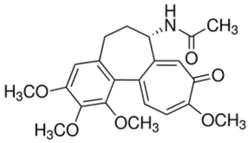 Colchicine (CAS Number: 64-86-8)View Details
Colchicine (CAS Number: 64-86-8)View Details
64-86-8 -
 ColchicineView Details
ColchicineView Details
64-86-8 -
 Colchicine API Phytochemical PowderView Details
Colchicine API Phytochemical PowderView Details
64-86-8