Triiron tetraoxide
Synonym(s):Fe NP NHS;FexOy
- CAS NO.:1317-61-9
- Empirical Formula: Fe3O4-2
- Molecular Weight: 231.53
- MDL number: MFCD00011010
- EINECS: 215-277-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-19 15:48:47
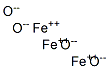
What is Triiron tetraoxide?
Description
Magnetite is a mineral whose primary component is an iron oxide that contains equal amounts of iron(II) and iron(III). Its empirical formula is Fe3O4, and it is often expressed as iron(II,III) oxide. In the past, it has been called ferrous–ferric oxide and triiron tetraoxide.
Magnetite is found in igneous, metamorphic, and sedimentary rocks. As its name implies, it is magnetic; it and other inherently magnetic iron-containing minerals are described as being ferrimagnetic. Magnetite is in the hexoctahedral crystal class; in the 3-D crystal structure, the purple atoms are iron, and the red atoms are oxygen.
Magnetite’s greatest use is as an important iron ore for steel manufacture. Other applications are as a catalyst in the Haber process for making ammonia, as a pigment for paints and ceramics, and as magnetic micro- and nanoparticles for a variety of processes and materials.
Studying an intriguing application of magnetite in nature, Lewis C. Naisbett-Jones, David L. G. Noakes, and colleagues at the University of North Carolina (Chapel Hill), LGL Ecological Research Associates (Bryan, TX), Oregon State University (Corvallis), and the Oregon Hatchery Research Center (Alsea) discovered that salmon use tiny magnetite crystals in their tissues to help them make their way from far out in the ocean back to their inland spawning locations.
The researchers subjected juvenile chinook salmon to strong magnetic pulses to show that the fish oriented their movements according to the direction of the magnetic field. Their results led the researchers to conclude that salmon are guided by Earth’s magnetic field to return to the rivers where they spawned.
Chemical properties
solid
Chemical properties
Magnetite (Fe3O4) constitutes the richest ore of iron; it is black and magnetic, one variety being called "lodestone".
The Uses of Triiron tetraoxide
Triiron tetraoxide can be used as pigment in paints, linoleum, ceramic glazes; in coloring glass; as a polishing compound; in the textile industry; in cathodes; as catalyst.
The Uses of Triiron tetraoxide
Mostly it is used as a black- pigment -catalyst in the Haber process and in the water gas shift reaction. It is used as a contrast-agent in MRI scanning, whereas it is used as a pigment in magnetic applications, polishing compounds, cosmetics, medicines, polymer, rubber, filler, building, construction, appliances, and magnetic inks.
The Uses of Triiron tetraoxide
Iron(II,III) oxide (Fe3O4) can be used as a heterogeneous catalyst for the Fenton type oxidation of rhodamine B. It can be used as an anode material for the fabrication of lithium-ion batteries. Fe3O4 can also be utilized in the catalysis of the oxygen reduction reaction (ORR) in the anion exchange membrane fuel cell.
General Description
Concentration 5mg/ml includes total weight nanocrystals plus ligands.
Flammability and Explosibility
Non flammable
Properties and Applications
|
TEST ITEMS |
SPECIFICATION |
|
APPEARANCE |
BLACK POWDER |
|
CONTENT OF Fe 3 O 4 |
90% min |
|
pH VALUE |
5-8 |
|
DENSITY |
4.5 g/ml |
|
SHADE |
CLOSE TO STANDARD |
|
OIL ABSORPTION |
10-20% |
|
RESIDUE ON 320 MESH |
0.3% max |
|
WATER SOLUBLE |
0.3% max |
|
VOLATITE 105 °C |
1.0% max |
|
TINTING STRENGTH |
98-102 % |
Properties of Triiron tetraoxide
| Melting point: | 1538 °C(lit.) |
| Density | 4.8-5.1 g/mL at 25 °C(lit.) |
| refractive index | 3.0 |
| Flash point: | 7 °C |
| storage temp. | -20°C |
| solubility | insoluble |
| solubility | Aqueous Acid (Slightly) |
| appearance | gray to black crystals or black powder |
| form | powder |
| color | black |
| Specific Gravity | 5.2 |
| Odor | at 100.00?%. odorless |
| Water Solubility | Insoluble in water and organic solvents. Soluble in concentrated mineral acids. |
| Merck | 14,4040 |
| Stability: | Stable. Incompatible with strong acids, chloroformates, peroxides. |
| CAS DataBase Reference | 1317-61-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Iron(ii,iii) oxide(1317-61-9) |
| EPA Substance Registry System | Iron oxide (Fe3O4) (1317-61-9) |
Safety information for Triiron tetraoxide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H304:Aspiration hazard H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H373:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P281:Use personal protective equipment as required. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Triiron tetraoxide
Triiron tetraoxide manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Iron(II, III) oxide CAS 1317-61-9View Details
Iron(II, III) oxide CAS 1317-61-9View Details
1317-61-9 -
 Iron(II, III) oxide CAS 1317-61-9View Details
Iron(II, III) oxide CAS 1317-61-9View Details
1317-61-9 -
 Iron(II, III) oxide CAS 1317-61-9View Details
Iron(II, III) oxide CAS 1317-61-9View Details
1317-61-9 -
 Iron Oxide(Ii,Iii),Magnetic Nanoparticles Solution CAS 1317-61-9View Details
Iron Oxide(Ii,Iii),Magnetic Nanoparticles Solution CAS 1317-61-9View Details
1317-61-9 -
 Iron(II,III) oxide nanopowder, APS 50-100 nm CAS 1317-61-9View Details
Iron(II,III) oxide nanopowder, APS 50-100 nm CAS 1317-61-9View Details
1317-61-9 -
 Iron(II,III) oxide CAS 1317-61-9View Details
Iron(II,III) oxide CAS 1317-61-9View Details
1317-61-9 -
 Iron Oxide CASView Details
Iron Oxide CASView Details -
 Iron oxide(II,III) magnetic nanopowder CASView Details
Iron oxide(II,III) magnetic nanopowder CASView Details