Clopidogrel Bisulfate
Synonym(s):(S)-Methyl (2-chlorophenyl)(6,7-dihydro-4H-thieno[3,2-c]pyridin-5-yl)acetate hydrogensulfate;(S)-(+)-Clopidogrel bisulfate;(S)-(+)-Methyl 2-(4,5,6,7-tetrahydrothieno[3,2-c]pyridin-5-yl)-2-(2-chlorophenyl)acetate hydrogensulfate;Clopidogrel Bisulphate;CLP
- CAS NO.:120202-66-6
- Empirical Formula: C16H16ClNO2S.H2O4S
- Molecular Weight: 419.9
- MDL number: MFCD00876395
- EINECS: 1806241-263-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
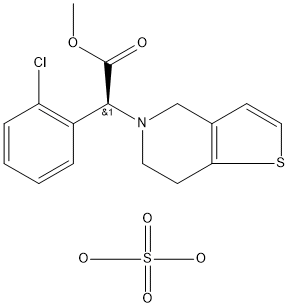
What is Clopidogrel Bisulfate?
Description
(S)-(+)-Clopidogrel is the functional enantiomer of clopidogrel and a prodrug whose thiol metabolite antagonizes purine binding to the platelet purinergic receptor P2Y12 (Ki = 316 nM in human platelets). It is metabolized by the cytochrome P450 (CYP) isoform CYP2C19 in rat liver and inhibits ADP-induced platelet aggregation ex vivo. Formulations containing (S)-(+)-clopidogrel have been used in combination with aspirin to prevent vascular ischemic events in patients with acute coronary syndromes.
Chemical properties
Off-White Solid
Originator
Iscover,Bristol-Myers
The Uses of Clopidogrel Bisulfate
Clopidogrel Bisulfate is an oral, thienopyridine class antiplatelet agent used to inhibit blood clots in coronary artery disease, peripheral vascular disease, and cerebrovascular disease.
The Uses of Clopidogrel Bisulfate
Used as an antithrombotic.
The Uses of Clopidogrel Bisulfate
An irreversible inhibitor of P2Y12.
Manufacturing Process
Levo-rotatory ammonium camphor-10-sulfonate is dissolved in a minimum of
water and applied to the column of Amberlite IRN-77 resin. Elution is carried
out with water. The eluted fractions containing the levo-rotatory camphor-10-
sulfonic acid are lyophilized, melting point 198°C.
32 g (0.0994 mole) of racemic methyl-α-5-(4,5,6,7-tetrahydro-thieno(3,2-
c)pyridyl)(2-chlorophenyl)-acetate are dissolved in 150 ml of acetone. 9.95 g
(0.0397 mole) of levo-rotatory camphor-10-sulfonic acid monohydrate are
added. The clear solution is left to stand at room temperature. After 48 hours
the reaction mixture is concentrated to 50 ml and left to stand at room
temperature for 24 hours. The obtained camphor-10-sulfonic acid salt of
methyl-α-5-(4,5,6,7-tetrahydro-thieno(3,2-c)pyridyl)(2-chlorophenyl)-acetate
(SR 25990) are filtered off, washed with acetone and dried (yield: 55% on the
basis of the starting racemate), melting point 165°C, [α]D20=+24.67 (c=1.58
g/100 ml; methanol). The crystals obtained above are redissolved in the
minimum of boiling acetone (50 ml). The crystals obtained after cooling are
filtered off, washed with acetone and dried (yield: 88%), m.p. 165°C,
[α]D20=+24.75 (c=1.68 g/100 ml; methanol).
12 g (0.022 mole) of the pure camphor-10-sulfonic acid salt of methyl-α-5-
(4,5,6,7-tetrahydro-thieno(3,2-c)pyridyl)(2-chlorophenyl)-acetate are
dissolved in a minimum of water. After cooling to 5°C, the aqueous solution
obtained is made alkaline with a saturated aqueous solution of sodium
hydrogen carbonate. The alkaline aqueous phase is extracted with
dichloromethane. The organic extracts are dried over anhydrous sodium
sulfate. On evaporation of the solvent a colorless oil of dextro-rotatory
methyl-α-5-(4,5,6,7-tetrahydro-thieno(3,2-c)pyridyl)(2-chlorophenyl)-acetate
is obtained (quantitative yield). Oil, [α]D20=+51.52 (c=1.61 g/100 ml;
methanol).
800 ml of a saturated aqueous solution of sodium bicarbonate are added to a
suspension of 200 g of SR 25990 in 800 ml of dichloromethane. After vigorous
shaking, the organic phase is separated, dried over sodium sulfate and the
solvent is removed under reduced pressure. The residue is dissolved in 500 ml
of ice-cold acetone and 20.7 ml of concentrated sulfuric acid (93.64%) are
added drop-wise. The precipitate formed is isolated by filtration and washed
with 1 L of acetone, then dried in a vacuum oven at 50°C. 139 g of pure
white crystals of hydrogen sulfate of dextro-rotatory methyl-α-5-(4,5,6,7-
tetrahydro-thieno(3,2-c)pyridyl)(2-chlorophenyl)-acetate (SR 25990 C) are
thus obtained, m.p. 184°C, [α]D20=+55.10 (c=1.891 g/100 ml; methanol).
brand name
Plavix (Sanofi Aventis).
Therapeutic Function
Platelet aggregation inhibitor
General Description
Clopidogrel bisulfate (CLP) is an antiplatelet drug, which belongs to the thienopyridine class of drug.
Biochem/physiol Actions
(S)-(+)-Clopidogrel hydrogen sulfate is an antithrombotic antiplatelet agent. It specifically and irreversibly inhibits the Purinoceptor P2Y12 subtype which inhibits ADP-induced platelet aggregation. (S)-(+)-Clopidogrel hydrogen sulfate is the active isomer.
Veterinary Drugs and Treatments
Clopidogrel, a platelet aggregation inhibitor, may be useful for preventing thrombi in susceptible cats. It may also improve pelvic limb circulation in cats after a cardiogenic embolic event via a vasomodulating effect secondary to inhibition of serotonin release from platelets. Research is ongoing.
Pharmacokinetics
Clopidogrel sulfate is a thienopyridine class inhibitor of platelet activation and aggregation through the binding the P2Y12 class of adenosine diphosphate (ADP) receptors on platelets. Clopidogrel is must be metabolized by CYP450-2C19 enzymes in liver to produce the thiol active metabolite antagonizes binding irreversibly to the P2Y12 receptor and the subsequent ADP-mediated activation of the glycoprotein GPIIb/IIIa complex, thereby inhibiting platelet aggregation.
Side Effects
During taking clopidogrel bisulfate, easy bleeding/bruising, stomach upset/pain, diarrhea, and constipation may occur.
Storage
Store at RT
Properties of Clopidogrel Bisulfate
| Melting point: | 174-176°C |
| storage temp. | 2-8°C |
| solubility | DMSO: ~26 mg/mL |
| form | solid |
| color | white |
| optical activity | [α]/D +54 to +70°, c = 0.5 in methanol |
| BRN | 9967887 |
| CAS DataBase Reference | 120202-66-6(CAS DataBase Reference) |
Safety information for Clopidogrel Bisulfate
Computed Descriptors for Clopidogrel Bisulfate
| InChIKey | FDEODCTUSIWGLK-RSAXXLAASA-N |
Clopidogrel Bisulfate manufacturer
PRAVEENLABORATORIES PVT LTD
Imex Overseas
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 CLOPIDOGREL BISULFATE 99%View Details
CLOPIDOGREL BISULFATE 99%View Details -
 Clopidogrel bisulphate 98%View Details
Clopidogrel bisulphate 98%View Details -
 Clopidogrel bisulphate 98%View Details
Clopidogrel bisulphate 98%View Details -
 Clopidogrel bisulphate 120202-66-6 98%View Details
Clopidogrel bisulphate 120202-66-6 98%View Details
120202-66-6 -
 120202-66-6 98%View Details
120202-66-6 98%View Details
120202-66-6 -
 S-(+)-Clopidogrel bisulfate 95% CAS 120202-66-6View Details
S-(+)-Clopidogrel bisulfate 95% CAS 120202-66-6View Details
120202-66-6 -
 Clopidogrel sulfate >98% (HPLC) CAS 120202-66-6View Details
Clopidogrel sulfate >98% (HPLC) CAS 120202-66-6View Details
120202-66-6 -
 Clopidogrel Bisulfate Powder API, IPView Details
Clopidogrel Bisulfate Powder API, IPView Details
120202-66-6
