cis-Anethol
Synonym(s):trans-1-Methoxy-4-(1-propenyl)benzene;trans-Anethole;4-Propenylanisole
- CAS NO.:104-46-1
- Empirical Formula: C10H12O
- Molecular Weight: 148.2
- MDL number: MFCD00009284
- EINECS: 203-205-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
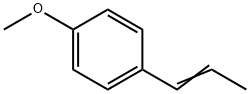
What is cis-Anethol?
Description
Anethole is the main component of anise, star anise and fennel oils. It is used in the food and cosmetic industries, in bleaching colors photography and as an embedding material. Is mainly a cause of intolerance to toothpaste but may cause contact dermatitis in food handlers.
Chemical properties
White crystals; sweet taste; odor of oil of anise. Affected by light. Soluble in 8 vol- umes of 80% alcohol, 1 volume of 90% alcohol; almost immiscible with water.
Chemical properties
Anethole occurs both as its (Z)-[25679-28-1] and (E)-[4180-23-8] isomers in nature; however, (E)-anethole is always the main isomer. Anethole occurs in anise oil (80–90%), star anise oil (>90%), and fennel oil (80%). (E)-Anethole forms colorless crystals (mp 21.5°C) with an anise-like odor and a sweet taste. Anethole is oxidized to anisaldehyde (e.g., with chromic acid); when hydrogenated, it is converted into l-methoxy-4-propylbenzene.
Physical properties
Appearance: this compound shows a colorless or light-yellow liquid appearance. Melting point: 20–21?°C. Solubility: dissolve in chloroform and ether in unlimited amount; soluble in benzene, ethyl acetate, acetone, carbon disulfide, petroleum ether, and alcohol; insoluble in water. It has a sweet smell.
History
In China, anise has been used as a traditional food spice and seasoning. In the anise
plant, the main biological active ingredient is volatile oils such as trans-anethole.
Till the end of last century, several methods to obtain the pure anethole have been
developed, including:
1. Cooling, crystallizing, and recrystallizing after the distillation of the anise oil.
2. Heating p-methoxyphenyl crotonic acid at 220–240?°C.
3. Heating and dehydrating the derived product of anisaldehyde and C2H5MgX.
4. Heating anisaldehyde together with propionic anhydride and sodium
propionate.
5. Adding concentrated hydrochloric acid and phosphoric acid to the mixture of
anisole and propionaldehyde at 0?°C and then heating the product with pyridine
to remove hydrogen chloride.
6. Prepare Grignard reagent using parabromoanisole, then react with allyl bromide
to produce p-methoxyphenylpropylene, then heat with potassium hydroxide, and
finally anethole was obtained after isomerization.
7. Using crystalline ferric chloride to catalyze the reaction of p-propenyl phenol
and methanol .
Anethole is easily oxidized when exposed in the air, especially in the presence of
heat, light, or catalyst . Therefore, in recent years, a series of studies have been
carried out on the synthesis of its derivatives in order to obtain more active
substances.
The Uses of cis-Anethol
Promote the white blood cells proliferation
Definition
ChEBI: A monomethoxybenzene that is methoxybenzene substituted by a prop-1-en-1-yl group at position 4.
Preparation
Production. Anethole is isolated from anethole-rich essential oils as well as from
sulfate turpentine oils or is synthesized starting from anisole.
1) Anethole can be obtained from oils in which it occurs as a major component
(main source is star anise oil) by distillation and/or crystallization.
2) A fraction of American sulfate turpentine oil (0.5% of the total) consists
mainly of an azeotropic mixture of anethole and caryophyllene. (E)-Anethole
can be isolated from this mixture by crystallization.
3) Another fraction of American sulfate turpentine oil (1% of the total) consists
essentially of an azeotropic mixture of estragole (l-methoxy-4-allylbenzene,
bp101.3 kPa 216°C) and α-terpineol. Treatment with potassium hydroxide
yields a mixture of anethole isomers and ??-terpineol, which can be separated
by fractional distillation.
4) Synthesis from anisole and propionic acid derivatives. Anisole is converted
into 4-methoxypropiophenone by Friedel–Crafts acylation with propionyl
chloride or propionic anhydride. The ketone is hydrogenated to the corresponding
alcohol with a copper chromite catalyst.The alcohol is dehydrated
in the presence of acidic catalysts to a (Z)-/(E)-mixture of anetholes.
General Description
White crystals or a liquid. Odor of anise oil and a sweet taste.
Air & Water Reactions
Slightly water soluble .
Reactivity Profile
Protect from light .
Health Hazard
ACUTE/CHRONIC HAZARDS: Toxic.
Fire Hazard
cis-Anethol is combustible.
Contact allergens
Anethole is the main component of anise, star anise, and fennel oils. It is used in perfumes, food and cosmetic industries (toothpastes), bleaching colors, and photography, and as an embedding material.
Pharmacology
Anethole is the main ingredient in star anise oil and possesses a variety of pharmacological effects.
1. Increasing the white blood cellular activity. Some agents such as Shengbaining
and Shengxuening, whose main active ingredients were extracted from the star
anise, can promote mature white blood cells in the bone marrow to spread into
the surrounding blood. Due to the body’s own feedback, mature and release
speed of bone marrow cells were accelerated. It can also keep bone marrow
cells’ activity, enhancing the white blood cells (especially granulocytes).
2. Bacteriostatic effect. Star anise oil shows antibacterial effects in a variety of
strains including Staphylococcus aureus, Escherichia coli, Bacillus subtilis,
Aspergillus niger, Aspergillus flavus, Penicillium citrinum, yeast, Shigella, diphtheria bacillus, and Salmonella typhi. The results laid a theoretical foundation for
the development and utilization of novel plant-derived antifungal propenylbenzene derivatives.
3. Antiviral effects . Star anise oil can act on different acyclovir-susceptible and
acyclovir-resistant herpes simplex virus type 1 (HSV-1) strains.
4. Other effects. Anethole can improve the activity of anticholinesterase . It
shows significant inhibitory effect against acetylcholinesterase and butyrylcholinesterase, and the IC50 value was 39.89±0.32?μg/mL and 75.35±1.47?μg/mL,
while the value for star anise oil was 36.00±0.44?μg/mL and 70.65±0.96?μg/
mL, respectively. It has also been reported that anethole has an antioxidant effect.
Through structural transformation, a variety of compounds can be prepared from
anethole. Anisaldehyde, produced by the oxidation reaction, was widely used in the
preparation of flavors for its lasting aroma. On the other hand, it can also be used as
the intermediate during the preparation of drugs such as amoxicillin.
Clinical Use
As a drug, anethole is mainly used for leukopenia caused by tumor chemotherapy.
Safety Profile
Poison by ingestion. Questionable carcinogen with experimental tumorigenic data. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes. See also ETHERS.
Properties of cis-Anethol
| Melting point: | 20-21 °C(lit.) |
| Boiling point: | 234-237 °C(lit.) |
| Density | 0.988 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 195 °F |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMSO: 30 mg/ml; PBS (pH 7.2): 10 mg/ml |
| form | Oil |
| color | Colorless to off-white |
| Odor | at 100.00 %. sweet anise licorice medicinal |
| Water Solubility | 148.2mg/L(25 ºC) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 104-46-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, 1-methoxy-4-(1-propenyl)-(104-46-1) |
| EPA Substance Registry System | Anethole (104-46-1) |
Safety information for cis-Anethol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for cis-Anethol
cis-Anethol manufacturer
Gem Aromatics Pvt Ltd
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

You may like
-
 104-46-1 98%View Details
104-46-1 98%View Details
104-46-1 -
 Anethole 98%View Details
Anethole 98%View Details
104-46-1 -
 trans-Anethole 98% (GC) CAS 104-46-1View Details
trans-Anethole 98% (GC) CAS 104-46-1View Details
104-46-1 -
 104-46-1 Anethole 99%View Details
104-46-1 Anethole 99%View Details
104-46-1 -
 104-46-1 99%View Details
104-46-1 99%View Details
104-46-1 -
 104-46-1 Anethole 98%View Details
104-46-1 Anethole 98%View Details
104-46-1 -
 Anethole CAS 104-46-1View Details
Anethole CAS 104-46-1View Details
104-46-1 -
 ANETHOLE For Synthesis CAS 104-46-1View Details
ANETHOLE For Synthesis CAS 104-46-1View Details
104-46-1
