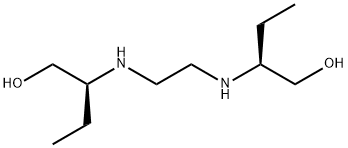
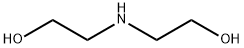
Ciclopirox ethanolamine
Synonym(s):6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone ethanolammonium salt
- CAS NO.:41621-49-2
- Empirical Formula: C14H24N2O3
- Molecular Weight: 268.36
- MDL number: MFCD00078997
- EINECS: 255-464-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-24 15:44:27
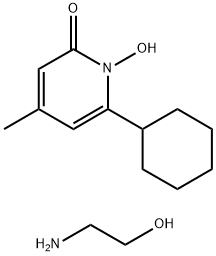
What is Ciclopirox ethanolamine?
The Uses of Ciclopirox ethanolamine
Ciclopirox olamine (Loprox, Penlac) is a synthetic, broad-spectrum hydroxypyridone antifungal agent. It is chemically unrelated to the imidazoles or any other antifungal agent currently available in the United States. It appears to act through intracellular depletion of substrates and/or ions principally by inhibition of transmembrane transport of these substances. It is active against dermatophytes, yeast, and Pityrosporum orbiculare. It also demonstrates activity against Trichomonas vaginalis, some mycoplasma, and some gram-positive and gram-negative bacteria.
Indications
Ciclopirox olamine (Loprox, Penlac) is a synthetic, broad-spectrum hydroxypyridone antifungal agent. It is chemically unrelated to the imidazoles or any other antifungal agent currently available in the United States. It appears to act through intracellular depletion of substrates and/or ions principally by inhibition of transmembrane transport of these substances. It is active against dermatophytes, yeast, and Pityrosporum orbiculare. It also demonstrates activity against Trichomonas vaginalis, some mycoplasma, and some gram-positive and gram-negative bacteria.
Manufacturing Process
Ciclopirox may be produced as follows: 2 g of 4-methyl-6-cyclohexyl-2-pyrone
were heated with 1 g of hydroxylamine hydrochloride and 5 g of 2-
aminopyridine to 80 C for 8 hours.
The reaction mixture was then dissolved in methylene chloride, the amine was
removed by shaking with dilute hydrochloric acid, the reaction product was
extracted from the organic phase by means of dilute sodium hydroxide
solution and the alkaline solution was acidified with acetic acid to a pH value
of 6. The 1-hydroxy-4-cnethyl-6-cyclohexyl-2-pyridone precipitated in
crystalline form. It was filtered off with suction, washed with water and dried.
The yield was 1.05 g (49% of theory); melting point 143 C.
Reaction of ciclopirox with ethanolamine gives the desired prod
Biological Activity
ciclopirox ethanolamine, working as an iron chelator, suppresses a substantial number of clinically relevant dermatophytes, yeasts, and molds, including the frequently azole-resistant candida species candida glabrata, candida krusei, and candida guilliermondii. moreover, ciclopirox has been proved to inhibit a wide range of bacteria in humans, including many gram-positive and gram-negative species pathogenic bacteria. [1]
Safety Profile
Poison by intravenous andintraperitoneal routes. Moderately toxic by ingestion andsubcutaneous routes. Experimental reproductive effects.When heated to decomposition it emits toxic fumes ofNOx.
Side Effects
Common side effects include local skin irritation, rash, dryness, and peeling. Severe allergic reactions are rare but require immediate medical attention.
Mechanism
Ciclopirox exerts its action by binding to and chelating trivalent cations, such as Fe3+ and Al3+, thereby inhibiting the availability of essential co-factors for enzymes. This may lead to a loss of activity of enzymes that are essential for cellular metabolism, organization of cell wall structure and other crucial cell functions. In addition, ciclopirox exerts its anti-inflammatory activity by inhibiting 5-lipoxygenase and cyclooxygenase (COX).
References
[1]niewerth m, kunze d, seibold m, schaller m, korting hc and hube b. ciclopirox olamine treatment affects the expression pattern of candida albicans genes encoding virulence factors, iron metabolism proteins, and drug resistance factors. antimicrob agents chem. 2003 jun; 47(6): 180517.
[2]linden t, katschinski dr, eckhardt k, scheid a, pagel h and wenger rh. the antimycotic ciclopirox olamine induces hif-1 stability, vegf expression, and angiogenesis. faseb. 2003 feb; 17: 761–3.
[3]subissi a, monti d, togni g and mailland f. recent nonclinical and clinical data relevant to its use as a topical antimycotic agent. drugs. 2010 nov; 70(16): 2133-52.
Properties of Ciclopirox ethanolamine
| Melting point: | 144 C |
| Density | 0.41g/cm3 at 25℃ |
| vapor pressure | 0-0Pa at 20-50℃ |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| solubility | Sparingly soluble in water, very soluble in ethanol (96 per cent) and in methylene chloride, slightly soluble in ethyl acetate, practically insoluble in cyclohexane. |
| form | neat |
| form | Solid |
| color | White to Off-White |
| CAS DataBase Reference | 41621-49-2(CAS DataBase Reference) |
Safety information for Ciclopirox ethanolamine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ciclopirox ethanolamine
Ciclopirox ethanolamine manufacturer
Kumar Organic Products Limited
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 41621-49-2 Ciclopirox olamine 99%View Details
41621-49-2 Ciclopirox olamine 99%View Details
41621-49-2 -
 41621-49-2 99%View Details
41621-49-2 99%View Details
41621-49-2 -
 Ciclopirox olamine 97% CAS 41621-49-2View Details
Ciclopirox olamine 97% CAS 41621-49-2View Details
41621-49-2 -
 Ciclopirox Olamine CAS 41621-49-2View Details
Ciclopirox Olamine CAS 41621-49-2View Details
41621-49-2 -
 Ciclopirox olamine CAS 41621-49-2View Details
Ciclopirox olamine CAS 41621-49-2View Details
41621-49-2 -
 Ciclopirox Olamine Powder, BP,USPView Details
Ciclopirox Olamine Powder, BP,USPView Details
29342-05-0 -
 Ciclopirox Olamine USP/ EP (Kopcyclamine), 41621-49-2View Details
Ciclopirox Olamine USP/ EP (Kopcyclamine), 41621-49-2View Details
41621-49-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
