Ciclopirox
Synonym(s):6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone;6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridinone;Ciclopirox;HOE 296b
- CAS NO.:29342-05-0
- Empirical Formula: C12H17NO2
- Molecular Weight: 207.27
- MDL number: MFCD00599441
- EINECS: 249-577-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
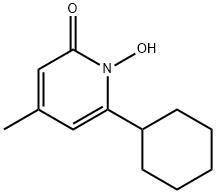
What is Ciclopirox?
Absorption
Rapidly absorbed after oral administration. Mean absorption of ciclopirox after application to nails of all twenty digits and adjacent 5 millimeters of skin once daily for 6 months in patients with dermatophytic onychomycoses was less than 5% of the applied dose. Ciclopirox olamine also penetrates into hair and through the epidermis and hair follicles into sebaceous glands and dermis.
Toxicity
Oral LD50 in rat is >10 ml/kg. Symptoms of overexposure include drowsiness and headache.
Chemical properties
White or Light-Yellow Powder
Originator
Fungirox Esmalte, UCI-Farma
The Uses of Ciclopirox
Broad spectrum antimycotic agent with some antibacterial activity.
The Uses of Ciclopirox
Ciclopirox (Penlac) is a synthetic antifungal agent. Ciclopirox (Penlac) is used for topical dermatologic treatment of superficial mycoses. Ciclopirox (Penlac) is most useful against Tinea versicolor. [1] In a study conducted to further elucidate ciclopir
Indications
Used as a topical treatment in immunocompetent patients with mild to moderate onychomycosis of fingernails and toenails without lunula involvement, due to Trichophyton rubrum.
Background
Ciclopirox olamine (used in preparations called Batrafen, Loprox, Mycoster, Penlac and Stieprox) is a synthetic antifungal agent for topical dermatologic treatment of superficial mycoses. In particular, the agent is especially effective in treating Tinea versicolor.
What are the applications of Application
Ciclopirox is a broad spectrum antimycotic agent
Definition
ChEBI: A cyclic hydroxamic acid that is 1-hydroxypyridin-2(1H)-one in which the hydrogens at positions 4 and 6 are substituted by methyl and cyclohexyl groups, repectively. A broad spectrum antigfungal agent, it also exhibits antibacterial acti ity against many Gram-positive and Gram-negative bacteria, and has anti-inflammatory properties. It is used a a topical treatment of fungal skin and nail infections.
Indications
Ciclopirox penetrates rapidly into and through the skin and nail keratin. After dermal application ofa1% cream formulation, about 1.3 % of the dose is absorbed. Serum levels range around 10 μg/L after application of a dose of 37 mg of ciclopirox. After vaginal administration, 15 – 20 % of the dose is absorbed. Ciclopirox penetrates well into the skin and nail matrix. Absorbed ciclopirox is mainly eliminated by the kidneys; 1.1 – 1.7 % of the topical dose is eliminated in the urine within four days. Only a fraction thereof is unaltered ciclopirox. Approximately 96% of the absorbed drug is bound to human serum proteins.
Manufacturing Process
A mixture of 5-oxo-3-methyl-5-cyclohexylpentene-2 acid 1-methyl ester and
5-oxo-3-methyl-5-cyclohexylpentene-3 acid 1-methyl ester was obtained by
condensation of hexahydrobenzoyl chloride with β,β-dimethylacrylic acid
methyl ester. 11.2 g of this mixture and a solution of 4.6 g of sodium acetate
and 4 g of hydroxylamine hydrochloride were shaken for 20 hours at 25°C
with a mixture of 8 ml of water and 15 ml methanol. Subsequently, a solution
of 4 g of sodium hydroxide in 8 ml of water was then added, while cooling,
shaken for 1 hour at room temperature. The mixture was extracted by means
of benzene and the aqueous phase was acidified to reach a pH of 6. 3.5 g of
1-hydroxy-4-methyl-6-cyclohexyl-2-pyridone were obtained; melting point
144°C.
For preparation of cyclopirox from 1-hydroxy-4-methyl-6-cyclohexyl-2-
pyridone was added 2-aminoethanol (1:1).Merck Index, Monograph number: 2325, Twelfth edition, 1996, Editor: S.
Budavari; Merck and Co., Inc.
Greene L.A.; US Patent No. 5,846,984; Dec. 8, 1998; Assigned to The
Trustees of Columbia University in the City of New York (New York, NY)
Lohaus G. et al.; US Patent No. 3,883,545; May 13, 1975; Assigned to
Hoechst Aktiengesellschaft, Frankfurt am Main, Germany
brand name
Loprox(Medicis); Penlac (Sanofi Aventis).
Therapeutic Function
Antifungal
Antimicrobial activity
Ciclopirox has a broad in vitro antifungal activity spectrum, including dermatophytes, yeasts, and moulds. The most important fungi within the activity spectrum of ciclopirox. Ciclopirox is primarily fungistatic, but if the contact time is long enough and the concentration exceeds 20 μg/mL, it exerts a fungicidal effect on yeasts and dermatophytes. In vitro, the effect is limited to proliferating fungi and is greatly dependent on the nutrient medium.
Mechanism of action
Ciclopirox is a substituted pyridione derivative. Experiments show that the mechanism of action involves a disruption of transport mechanisms into the cell plasma, especially the uptake of leucine. Further, the uptake of other amino acids such as phenylalanine and lysine, as well as the uptake of potassium and phosphate ions is impaired. This all probably involves a change in membrane permeability, as reported for some other antimycotics, although ciclopirox does not show lytic activity. Consecutively, the substance significantly reduces fungal growth rates.
Mechanism of action
Ciclopirox has a unique mechanism of action through chelation of polyvalent cations, such as Fe3+, which causes inhibition of a number of metal-dependent enzymes within the fungal cell.
Pharmacokinetics
Ciclopirox is a broad-spectrum antifungal medication that also has antibacterial and anti-inflammatory properties. Its main mode of action is thought to be its high affinity for trivalent cations, which inhibit essential co-factors in enzymes. Ciclopirox exhibits either fungistatic or fungicidal activity in vitro against a broad spectrum of fungal organisms, such as dermatophytes, yeasts, dimorphic fungi, eumycetes, and actinomycetes. In addition to its broad spectrum of action, ciclopirox also exerts antibacterial activity against many Gram-positive and Gram-negative bacteria. Furthermore, the anti-inflammatory effects of ciclopirox have been demonstrated in human polymorphonuclear cells, where ciclopirox has inhibited the synthesis of prostaglandin and leukotriene. Ciclopirox can also exhibit its anti-inflammatory effects by inhibiting the formation of 5-lipoxygenase and cyclooxygenase.
Clinical Use
Ciclopirox is a hydroxylated pyridinone that is employed for superficial dermatophytic infections, principally onychomycosis.
Side Effects
Topically applied, ciclopirox is well tolerated. The incidence of local irritations such as itching and burning sensations range between 1 and 4 % and are often due to the galenic formulation.
Metabolism
Glucuronidation is the main metabolic pathway of ciclopirox.
storage
Store at +4°C
Properties of Ciclopirox
| Melting point: | 1440C |
| Boiling point: | 350.0±25.0 °C(Predicted) |
| Density | 1.193±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Slightly soluble in water, freely soluble in anhydrous ethanol and in methylene chloride |
| form | Solid |
| pka | 6.25±0.58(Predicted) |
| color | Off-White |
| CAS DataBase Reference | 29342-05-0(CAS DataBase Reference) |
Safety information for Ciclopirox
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ciclopirox
Ciclopirox manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 Ciclopirox 98%View Details
Ciclopirox 98%View Details -
 29342-05-0 99%View Details
29342-05-0 99%View Details
29342-05-0 -
 Ciclopirox 99%View Details
Ciclopirox 99%View Details
29342-05-0 -
 Ciclopirox 97.00% CAS 29342-05-0View Details
Ciclopirox 97.00% CAS 29342-05-0View Details
29342-05-0 -
 Ciclopirox CAS 29342-05-0View Details
Ciclopirox CAS 29342-05-0View Details
29342-05-0 -
 Ciclopirox 98% (HPLC) CAS 29342-05-0View Details
Ciclopirox 98% (HPLC) CAS 29342-05-0View Details
29342-05-0 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1