Chlorzoxazone
Synonym(s):5-Chloro-2(3H)-benzoxazolone;5-Chloro-2-hydroxybenzoxazole;Chlorzoxazone;CLZ
- CAS NO.:95-25-0
- Empirical Formula: C7H4ClNO2
- Molecular Weight: 169.57
- MDL number: MFCD00005717
- EINECS: 202-403-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
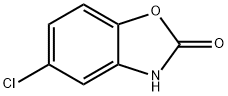
What is Chlorzoxazone?
Toxicity
Oral, mouse: LD50 = 440 mg/kg; Oral, rat: LD50 = 763 mg/kg; Symptoms of overdose include diarrhea, dizziness, drowsiness, headache, light-headedness, nausea, and vomiting.
Description
Chlorzoxazone is a muscle relaxant. It acts by blocking nerve impulses or pain sensations that are sent to brain. Typically, it is used together with rest and physical therapy to treat skeletal muscle conditions such as pain or injury. Chlorzoxazone, 5-chloro-2-benzoxazolione, is synthesized by a heterocyclization reaction of 2-amino-4-chlorophenol with phosgene. Skeletal muscle relaxants have conventionally been classified into one group; however, they are actually a heterogeneous group of medications commonly used to treat two different types of underlying conditions – spasticity from upper motor neuron syndromes and muscular pain or spasms from peripheral musculoskeletal conditions. Medications classified as skeletal muscle relaxants are baclofen, carisoprodol, chlorzoxazone, cyclobenzaprine, dantrolene, metaxalone, methocarbamol, orphenadrine, and tizanidine. These drugs may impair mental and/or physical abilities required for driving vehicles. As a class, skeletal muscle relaxants have central nervous system (CNS)-related side effects: drowsiness, dizziness, decreased alertness, blurred vision, and ataxia. Their use has been associated with a twofold increase in the risk for motor vehicle crashes. Muscle relaxants are included in the ‘Beers List’ of potentially inappropriate medications in older adults. Most muscle relaxants are poorly tolerated by elderly patients because they cause anticholinergic adverse effects, sedation, and weakness. Although extremely uncommon, this compound may yield to idiosyncratic and unpredictable type of liver toxicity. The concomitant use of alcohol or other CNS depressants may have an additive effect. Individuals on chlorzoxazone containing drugs should be monitored for abnormal liver enzymes (e.g., aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase, bilirubin, etc.).
Description
Chlorzoxazone is a centrally acting muscle relaxant and activator of small and intermediate conductance calcium-activated potassium channels (EC50s = 87 and 98 μM for KCa2.2 and KCa3.1, respectively). In vivo, chlorzoxazone (10 mg/kg) decreases alcohol but not water intake in a dose-dependent manner and reduces the propensity for rapid initial alcohol intake in rats with intermittent, but not continuous, access to alcohol. Formulations containing chlorzoxazone have been used in the treatment of pain and stiffness caused by muscle spasm. This product is also available as an analytical reference standard .
Description
Chlorzoxazone (Item No. 25826) is an analytical reference standard categorized as a skeletal muscle relaxant. This product is intended for analytical forensic applications. This product is also available as a general research tool .
Chemical properties
White to Off-White Solid
Originator
Paraflex ,McNeil, US ,1958
The Uses of Chlorzoxazone
Chlorzoxazone is a benzoxazolone derivative that causes skeletal muscle relaxation and blocks spasticity in clinical studies. Chlorzoxazone enhances small and intermediate conductance calcium-activated potassium channels (EC50s = 87 and 98 μM for KCa2.2 and KCa3.1, respectively). The cytochrome P450 isoform CYP2E1 converts chlorzoxazone to 6-hydroxy chlorzoxazone . The urinary excretion of this 6-hydroxy metabolite is often used as a probe of CYP2E1 activity in studies of hepatotoxicity.
The Uses of Chlorzoxazone
Muscle relaxant (skeletal)
The Uses of Chlorzoxazone
For the relief of discomfort associated with acute painful musculoskeletal conditions.
Background
A centrally acting central muscle relaxant with sedative properties. It is claimed to inhibit muscle spasm by exerting an effect primarily at the level of the spinal cord and subcortical areas of the brain. (From Martindale, The Extra Pharmacopoea, 30th ed, p1202)
Indications
For the relief of discomfort associated with acute painful musculoskeletal conditions.
What are the applications of Application
Chlorzoxazone is a skeletal muscle relaxant
Definition
ChEBI: A member of the class of 1,3-benzoxazoles that is 1,3-benzoxazol-2-ol in which the hydrogen atom at position 5 is substituted by chlorine. A centrally acting muscle relaxant with sedative properties, it is used for the symptomatic treatment of painful mus le spasm.
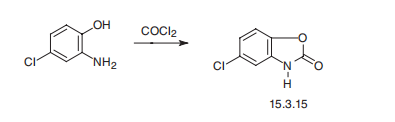
Manufacturing Process
A solution of 16.9 g (0.1 mol) of 2-amino-5-chlorobenzoxazole in 200 ml of 1
N HCl is refluxed until precipitation is complete. The resulting solid is collected
by filtration, dissolved in 200 ml of 1 N NaOH and the solution extracted with
50 ml of ether. Acidification of the alkaline solution gives a precipitate which is
purified by crystallization from acetone to give 2-hydroxy-5-chlorobenzoxazole
melting at 191° to 191.5°C.
brand name
Paraflex (Ortho-McNeil).
Therapeutic Function
Muscle relaxant
General Description
Chlorzoxazone is a centrally active muscle relaxant used to treat painful muscle spasms linked to musculoskeletal disorders, such as fibrositis, bursitis, myositis, spondylitis, etc.
Pharmaceutical secondary standard for application in quality control. Provides pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Biochem/physiol Actions
Chlorzoxazone is a skeletal muscle relaxant.
Pharmacokinetics
Chlorzoxazone is a centrally-acting agent for painful musculoskeletal conditions. Data available from animal experiments as well as human study indicate that chlorzoxazone acts primarily at the level of the spinal cord and subcortical areas of the brain where it inhibits multisynaptic reflex a.c. involved in producing and maintaining skeletal muscle spasm of varied etiology. The clinical result is a reduction of the skeletal muscle spasm with relief of pain and increased mobility of the involved muscles.
Synthesis
Chlorzoxazone, 5-chloro-2-benzoxazolione (15.3.15), is synthesized by a hetercyclization reaction of 2-amino-4-chlorphenol with phosgene.
Environmental Fate
Chlorzoxazone ismetabolized by the CYP1A2 and CYP2E1 microsomal enzymes to a toxic metabolite 6-hydroxychlorzoxazone.
Metabolism
Chlorzoxazone is rapidly metabolized in the liver and is excreted in the urine, primarily in a conjugated form as the glucuronide.
Toxicity evaluation
Chlorzoxazone is a white to off-white solid. It is soluble in organic solvents such as ethanol, methanol, and dimethyl sulfoxide (DMSO).
Properties of Chlorzoxazone
| Melting point: | 191-192 °C (lit.) |
| Density | 1.3771 (rough estimate) |
| refractive index | 1.5557 (estimate) |
| storage temp. | room temp |
| solubility | DMSO: soluble50mg/mL, clear |
| pka | pKa 8.3 (Uncertain) |
| form | Powder |
| color | white to off-white |
| Water Solubility | 0.1 g/100 mL |
| Merck | 14,2194 |
| CAS DataBase Reference | 95-25-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Chlorzoxazone(95-25-0) |
| EPA Substance Registry System | Chlorzoxazone (95-25-0) |
Safety information for Chlorzoxazone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Chlorzoxazone
Chlorzoxazone manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



![3-(5-CHLORO-2-OXOBENZO[D]OXAZOL-3(2H)-YL)PROPANENITRILE](https://img.chemicalbook.in/StructureFile/ChemBookStructure3/GIF/CB1465208.gif)
You may like
-
 Chlorzoxazone 99%View Details
Chlorzoxazone 99%View Details -
 Chlorzoxazone 99%View Details
Chlorzoxazone 99%View Details -
 Chlorzoxazone 98%View Details
Chlorzoxazone 98%View Details -
 Chlorzoxazone 99%View Details
Chlorzoxazone 99%View Details -
 Chlorzoxazone 97% CAS 95-25-0View Details
Chlorzoxazone 97% CAS 95-25-0View Details
95-25-0 -
 Powder Chlorzoxazone Chemical, Usage/Application: LaboratoryView Details
Powder Chlorzoxazone Chemical, Usage/Application: LaboratoryView Details
95-25-0 -
 Chlorzoxazone Powder API MANUFACTURER INDIAView Details
Chlorzoxazone Powder API MANUFACTURER INDIAView Details
95-25-0 -
 Chlorzoxazone IP PowderView Details
Chlorzoxazone IP PowderView Details
95-25-0
