Cefazolin sodium salt
- CAS NO.:27164-46-1
- Empirical Formula: C14H15N8NaO4S3
- Molecular Weight: 478.5
- MDL number: MFCD00056883
- EINECS: 248-278-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-04 20:40:58
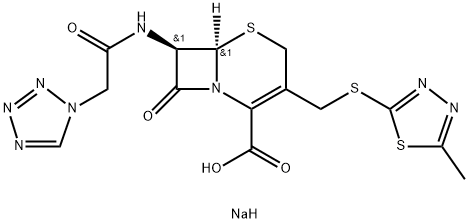
What is Cefazolin sodium salt?
Description
Cefazolin was synthesized by Fujisawa Pharmaceutical Co. in 1969. It was the first of the cephem antibiotics to introduce a thiadiazolylthiomethyl group at the 3 position and a tetrazole group at the 7 position in the side chain. Cefazolin is a parenteral cephem antibiotic showing better activity against gramnegative bacteria than cephalothin or cephaloridine. Its bactericidal activity, tissue distribution, and urinary excretion are excellent and it has wide clinical use.
Chemical properties
White to off-white powder
Originator
Cefamedin,Fujisawa,Japan,1971
The Uses of Cefazolin sodium salt
Semi-synthetic antibiotic derived from 7-amino-cepphalosporanic acid. An antibacterial
The Uses of Cefazolin sodium salt
enzyme inhibitor, Gaucher's disease therapy
The Uses of Cefazolin sodium salt
Semi-synthetic antibiotic derived from 7-amino-cephalosporanic acid. An antibacterial.
What are the applications of Application
Cefazolin sodium salt is an inhibitor of bacterial cell wall synthesis
Definition
ChEBI: A cephalosporin organic sodium salt having [(5-methyl-1,3,4-thiadiazol-2-yl)sulfanyl]methyl and (1H-tetrazol-1-ylacetyl)amino side-groups.
Manufacturing Process
7-Aminocephalosporanic acid is converted to its sodium salt and acylated with 1H-tetrazole-1-acetyl chloride. The acetoxy group is then displaced by reaction with 5-methyl-1,3,4-thiadiazole-2-thiol in buffer solution. The product acid is converted to the sodium salt by NaHCO3.
brand name
Ancef (GlaxoSmithKline); Kefzol (Lilly).
Therapeutic Function
Antibacterial
Biological Activity
cefazolin is a semisynthetic antibiotic with a broad spectrum of antibacterial activity. cefazolin has exhibited high activity against gram-positive bacteria and gram-negative bacteria [1].
Clinical Use
Cefazolin (Ancef, Kefzol) is one of a series of semisyntheticcephalosporins in which the C-3 acetoxy function has beenreplaced by a thiol-containing heterocycle—here, 5-methyl-2-thio-1,3,4-thiadiazole. It also contains the somewhatunusual tetrazolylacetyl acylating group. Cefazolin wasreleased in 1973 as a water-soluble sodium salt. It is activeonly by parenteral administration.
Cefazolin provides higher serum levels, slower renalclearance, and a longer half-life than other first-generationcephalosporins. It is approximately 75% protein bound inplasma, a higher value than for most other cephalosporins.Early in vitro and clinical studies suggest that cefazolin ismore active against Gram-negative bacilli but less activeagainst Gram-positive cocci than either cephalothin orcephaloridine. Occurrence rates of thrombophlebitis followingintravenous injection and pain at the site of intramuscularinjection appear to be the lowest of the parenteralcephalosporins.
Veterinary Drugs and Treatments
In the United States, there are no cefazolin products approved for veterinary species but it has been used clinically in several species when an injectable, first generation cephalosporin is indicated. It is used for surgical prophylaxis, and for variety of systemic infections (including orthopedic, soft tissue, sepsis) caused by susceptible bacteria. Most commonly given every 6 – 8 hours via parenteral routes, cefazolin constant rate intravenous infusion protocols are being developed as cefazolin is a time (above MIC)-dependent antibiotic, and serum/tissue concentrations can remain above MIC.
in vitro
in cultured mg-63 human osteosarcoma cell line, cefazolin (100 μg/ml) showed little or no effect on osteoblast replication. cefazolin (200μg/ml) significantly decreased cell replication, and 10,000 μg/ml caused cell death [2].
in vivo
in patients with normal and various degrees of compromised renal function, administration of cefazolin significantly decreased the urinary concentration and percentage of the dose excreted in the urine [3]. the half-life of cefazolin in serum of normal persons was 1.9 hr and as long as 35 hr in severely uremic patients. in uremic patients, cefazolin was well tolerated [4].
References
[1] kariyone k, harada h, kurita m, et al. cefazolin, a new semisynthetic cephalosporin antibiotic. i[j]. the journal of antibiotics, 1970, 23(3): 131-136.
[2] edin m l, miclau t, lester g e, et al. effect of cefazolin and vancomycin on osteoblasts in vitro[j]. clinical orthopaedics and related research, 1996, 333: 245-251.
[3] levison m e, levison s p, ries k, et al. pharmacology of cefazolin in patients with normal and abnormal renal function[j]. journal of infectious diseases, 1973, 128(supplement 2): s354-s357.
[4] craig w a, welling p g, jackson t c, et al. pharmacology of cefazolin and other cephalosporins in patients with renal insufficiency[j]. journal of infectious diseases, 1973, 128(supplement 2): s347-s353.
Properties of Cefazolin sodium salt
| Melting point: | 190 °C |
| refractive index | 20 ° (C=10, H2O) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | H2O: 50 mg/mL, clear, colorless |
| form | crystalline powder |
| color | White to Off-White |
| Sensitive | Light Sensitive |
| Merck | 14,1917 |
| BRN | 3585038 |
| Stability: | Stable, but may be heat sensitive - store in cool conditions. May discolour upon exposure to light - store in the dark. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 27164-46-1(CAS DataBase Reference) |
Safety information for Cefazolin sodium salt
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P342+P311:IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. |
Computed Descriptors for Cefazolin sodium salt
| InChIKey | LJIOGPYKTPCRAP-MVOALHSDNA-N |
| SMILES | C(C1=C(CS[C@]2([H])[C@H](NC(=O)CN3N=NN=C3)C(=O)N12)CSC1=NN=C(C)S1)(=O)O.[NaH] |&1:5,7,r| |
Cefazolin sodium salt manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran



You may like
-
 Cefazolin sodium sterile 98%View Details
Cefazolin sodium sterile 98%View Details -
 27164-46-1 98%View Details
27164-46-1 98%View Details
27164-46-1 -
 Cefazolin sodium salt 95% CAS 27164-46-1View Details
Cefazolin sodium salt 95% CAS 27164-46-1View Details
27164-46-1 -
 Cefazolin sodium sterile 99%View Details
Cefazolin sodium sterile 99%View Details -
 Cefazolin Sodium Salt CAS 27164-46-1View Details
Cefazolin Sodium Salt CAS 27164-46-1View Details
27164-46-1 -
 Cefazolin sodium sterile 98%View Details
Cefazolin sodium sterile 98%View Details -
 Cefazolin sodium salt CAS 27164-46-1View Details
Cefazolin sodium salt CAS 27164-46-1View Details
27164-46-1 -
 Cefazolin sodium salt CAS 27164-46-1View Details
Cefazolin sodium salt CAS 27164-46-1View Details
27164-46-1
