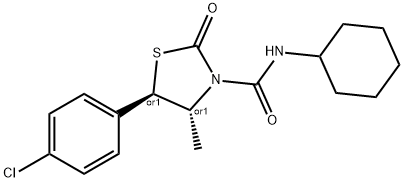
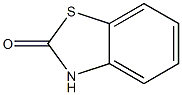
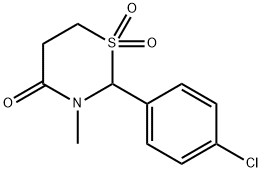
Chlormezanone
- CAS NO.:80-77-3
- Empirical Formula: C11H12ClNO3S
- Molecular Weight: 273.74
- MDL number: MFCD00143951
- EINECS: 201-307-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-12 07:03:40
What is Chlormezanone?
Toxicity
Symptoms of overdose include drowsiness, weakness, nausea, dizziness, abdominal pain, cerebral oedema and renal tubular necrosis, hyperglycaemia and hypoglycaemia, liver damage, encephalopathy, coma and death.
Originator
Trancopal,Winthrop-Breon,US,1958
The Uses of Chlormezanone
Chlormezanone improves the emotional state of the patient, relieving moderate anxiety and stress. However, it has a number of side effects, and because it does not have any advantage over other anxiolytics, it is rarely used in practice.
The Uses of Chlormezanone
Skeletal muscle relaxant
The Uses of Chlormezanone
The use of chlormezanone is associated with large increases in the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis.
Background
A non-benzodiazepine that is used in the management of anxiety. It has been suggested for use in the treatment of muscle spasm.
What are the applications of Application
Chlormezanone is a compound acting at the benzodiazapine site of GABAA receptors
Indications
Used in the management of anxiety and in the treatment of muscle spasm.
Definition
ChEBI: A 1,3-thiazine that is 1,3-thiazinan-4-one S,S-dioxide in which a hydrogen at position 2 is substituted by a 4-chlorophenyl group and the hydrogen attached to the nitrogen is substituted by methyl. A non-benzodiazepine muscle relaxant, it was used in the management of anxiety and in the treatment of muscle spasms until being discontinued worldwide by its manufacturer in 1996, due to rare but serious cutaneous reactions.
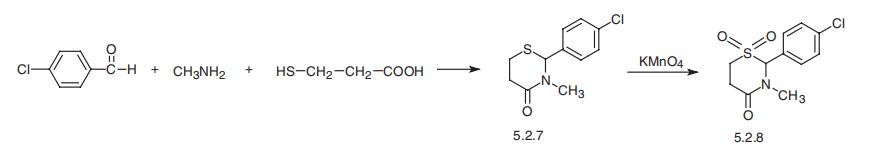
Manufacturing Process
A solution of 4-chlorobenzaldehyde is reacted with beta-mercaptopropionic
acid and with methylamine. The mixture is refluxed in benzene and water is
removed from an overhead separator. The reaction mixture was cooled,
washed with dilute ammonium hydroxide and water, and the benzene was
removed by distillation in vacuo. The oily residue was taken up in ether from
which it crystallized. The precipitate was recrystallized twice from ether to
yield 2-(4-chlorophenyl)-3-methyl-4-metathiazanone.
A solution of 11.2 g of potassium permanganate in 100 ml of warm water was
added dropwise to a well stirred solution of 10 g of 2-(4-chlorophenyl)-3-
methyl-4-metathiazanone in 50 ml of glacial acetic acid. The temperature was
kept below 30°C with external cooling. An aqueous sodium bisulfite solution
was then added to remove the manganese dioxide. The thick whitish oil which
separated was taken up in chloroform and the extract was washed with water.
Removal of the chloroform by distillation in vacuo yielded an oily residue
which solidified. The solid was recrystallized from isopropyl alcohol to give 5 gof the product, 2-(4-chlorophenyl)-3-methyl-4-metathiazanone-1,1-dioxide,
MP 116.2° to 118.6°C (corr.).
brand name
Trancopal (Sanofi Aventis).
Therapeutic Function
Tranquilizer
World Health Organization (WHO)
Chlormezanone is a sedative with antianxiety properties and a central skeletal muscle relaxant effect. It had already been falling into obsolescence for several years.
Biological Activity
Anxiolytic and skeletal muscle relaxant that acts at the benzodiazepine site of GABA A receptors.
Pharmacokinetics
Chlormezanone is a non-benzodiazepine muscle relaxant. It was discontinued worldwide in 1996 by its manufacturer due to confirmed serious and rare cutaneous reactions (toxic epidermal necrolysis).
Synthesis
Chlormezanone, 2-(p-chlorophenyl)-tetrahydro-3-methyl-4H-1,3- tiazin-4-on-1,1-dioxide (5.2.8), is synthesized by joint condensation of mercaptopropionic acid, methylamine, and 4-chlorobenzaldehyde, evidently through the intermediate stage of formation of 4-chlorobenzylidenemethylamine, giving the aminothioacetal 2- (p-chlorophenyl)-tetrahydro-3-methyl-4H-1,3-tiazin-4-one (5.2.7). Oxidation of the sulfur atom using potassium permanganate gives chlormezanone (5.2.8) [62,63].
Metabolism
Not Available
storage
Room temperature
Properties of Chlormezanone
| Melting point: | 114 °C |
| Boiling point: | 534.5±50.0 °C(Predicted) |
| Density | 1.2205 (rough estimate) |
| refractive index | 1.6300 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | -2.37±0.40(Predicted) |
| color | White to Pale Yellow |
| Merck | 14,2106 |
| NIST Chemistry Reference | Chlormezanone(80-77-3) |
Safety information for Chlormezanone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P330:Rinse mouth. P501:Dispose of contents/container to..… |
Computed Descriptors for Chlormezanone
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
 Chlormezanone CAS 80-77-3View Details
Chlormezanone CAS 80-77-3View Details
80-77-3 -
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3