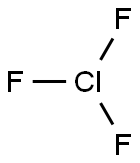
CHLORINE PENTAFLUORIDE
- CAS NO.:13637-63-3
- Empirical Formula: ClF5
- Molecular Weight: 130.45
- MDL number: MFCD00042566
- EINECS: 237-123-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
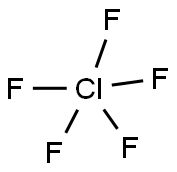
What is CHLORINE PENTAFLUORIDE?
Description
The gas chlorine pentafluoride (ClF5) was first prepared by D. F. Smith in 1963 by the reaction of ClF3?with F2?under heat and pressure. It was previously an unknown compound. Ironically, it was predicted on the basis of the existence of XeF4, which at the time was considered to be a novelty because it was believed that noble gases could not bind to other elements. ClF5?has occasionally been tried as an oxidant, but the HCl and HF byproducts make this a bad idea.
Chemical properties
colorless gas; critical temp 142.6°C; enthalpy of vaporization 22.21 kJ/mol; specific conductivity 1.25×10?9 ohm· cm [KIR78]
The Uses of CHLORINE PENTAFLUORIDE
Chlorine pentafluoride does not have anysignificant commercial application. It is usedas a fluorinating and oxidizing agent.
General Description
A colorless gas with a sweet odor. Toxic by inhalation and an irritant to skin, eyes and mucus membranes. Corrosive. Heavier than air. Under prolonged exposure to fire or intense heat the containers may violently rupture or rocket. Used as an oxidizer in propellants.
Air & Water Reactions
Reacts with water or moisture in the air to produce corrosive hydrofluoric acid and toxic chloride gas. Interaction with ice at -100°C, or with water vapor above 0°C is extremely vigorous (Christe, K.O. Inorg. Chem. 1972 11, 1220).
Reactivity Profile
CHLORINE PENTAFLUORIDE is a strong oxidizing agent. Nonflammable, but likely to react vigorously on contact with combustible materials. Reacts violently with lithium, calcium. Emits highly toxic fluoride and chloride fumes when heated to decomposition. Extremely vigorous reaction with water, steam, even ice [Pilipovich D. et al., Inorg. Chem., 1967, 6, p. 1918]. Very vigorous reaction with anhydrous nitric acid even at -100° C [Christie, K. O., Inorg. Chem. 1992, 11, p. 1220].
Health Hazard
TOXIC; may be fatal if inhaled or absorbed through skin. Fire will produce irritating, corrosive and/or toxic gases. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Runoff from fire control may cause pollution.
Health Hazard
Chlorine pentafluoride is a highly toxic gas.It is a severe irritant to the eyes, skin,and mucous membranes. Exposure can causelacrimation, corneal damage, skin burn, andlung damage. Other symptoms are nausea,vomiting, and dyspnea. The liquid is highlycorrosive to skin, causing painful burns.
LC50 value, inhalation (rats): 122 ppm/h.
Fire Hazard
Substance does not burn but will support combustion. Vapors from liquefied gas are initially heavier than air and spread along ground. These are strong oxidizers and will react vigorously or explosively with many materials including fuels. May ignite combustibles (wood, paper, oil, clothing, etc.). Some will react violently with air, moist air and/or water. Cylinders exposed to fire may vent and release toxic and/or corrosive gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
Safety Profile
Poison by inhalation. A corrosive material. Vigorous reaction in contact with water or anhydrous nitric acid. Violent reaction on contact with metals. When heated to decomposition it emits very toxic fumes of Cland F-. See also CHLORINE, FLUORINE, FLUORIDES, and CHLORINE TRIFLUORIDE.
Properties of CHLORINE PENTAFLUORIDE
| Melting point: | -103°C |
| Boiling point: | -13,1°C |
| Density | 1.774 (estimate) |
| form | colorless gas |
| color | colorless |
| Exposure limits | TLV-TWA 2.5 mg(F)/m3 (ACGIH, MSHA,
and OSHA). |
| EPA Substance Registry System | Chlorine fluoride (ClF5) (13637-63-3) |
Safety information for CHLORINE PENTAFLUORIDE
Computed Descriptors for CHLORINE PENTAFLUORIDE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1