Silicon Tetrafluoride
Synonym(s):Silicon(IV) fluoride
- CAS NO.:7783-61-1
- Empirical Formula: F4Si
- Molecular Weight: 104.08
- MDL number: MFCD00040533
- EINECS: 232-015-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
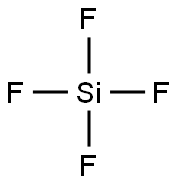
What is Silicon Tetrafluoride?
Chemical properties
A colorless, nonflammable, corrosive and toxic gas. Sharp, pungent suffocating odor, similar to hydrogen chloride. Vapor is heavier than air; density53.6.
Chemical properties
Silicon tetrafluoride exhibits a very pungent odor. Silicon tetrachloride has a suffocating odor and is moisture sensitive.
Physical properties
Colorless gas; very pungent odor; fumes heavily in moist air; density of the gas 4.69 g/L; heavier than air, density in air 3.5 (air = 1); sublimes at -95.7°C; solidifies at -90.2°C (under pressure); critical pressure 50atm; decomposes in water forming silicic acid and hydrofluoric acid.
The Uses of Silicon Tetrafluoride
Manufacture of fluosilicic acid, intermediate in manufacture of pure silicon, to seal water out of oil wells during drilling.
Preparation
Silicon tetrafluoride is prepared by heating silica with dilute hydrofluoric acid at high temperatures:
SiO2 + 4HF → SiF4 + 2H2O
Also, the tetrafluoride may be obtained by heating the elements:
Si + 2F2 → SiF4.
General Description
A colorless, nonflammable, corrosive and toxic gas with a pungent odor similar to that of hydrochloric acid. Very toxic by inhalation. Vapor is heavier than air. Under prolonged exposure to heat the containers may rupture violently and rocket.
Air & Water Reactions
Fumes in air. Decomposed exothermically by water or moisture in the air to hydrofluoric acid and silicic acid [Merck 11th ed. 1989; Handling Chemicals Safely 1980 p. 821].
Reactivity Profile
When heated to decomposition SILICON TETRAFLUORIDE emits toxic fluoride fumes [Lewis, 3rd ed., 1993, p. 1138]. Reacts violently with alcohols to form HF. Attacks many metals in the presence of moisture. Reacts violently to explosively with lithium nitride [Porritt, L. J., Chem. Brit., 1979, 15, p. 282]. Mixtures with sodium are shock-sensitive explosives [Leleu, Cahiers, 1975, (79), p. 270].
Hazard
Toxic by inhalation, strong irritant to mucous membranes.
Health Hazard
TOXIC; may be fatal if inhaled, ingested or absorbed through skin. Vapors are extremely irritating and corrosive. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Some may burn but none ignite readily. Vapors from liquefied gas are initially heavier than air and spread along ground. Some of these materials may react violently with water. Cylinders exposed to fire may vent and release toxic and/or corrosive gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
Safety Profile
A poison. Moderately toxic by inhalation. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of F-. See also FLUORIDES and HYDROFLUORIC ACID
Potential Exposure
Silicon tetrafluoride is not used in industry but may be a discharge byproduct of certain processes, including ore refining and smelting operations. It has been used in the manufacture of silane and fluosilicic acid, and pure electronic silicon.
Shipping
UN1859 Silicon tetrafluoride, Hazard Class: 2.3; Labels 2.3-Poisonous gas; 8-Corrosive material; Inhalation Hazard Zone B. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.
Incompatibilities
Water and air reactive Fluorides form explosive gases on contact with strong acids, acid fumes, and sodium. Water and air reactive. Corrosive. Fumes in air. Decomposed exothermically by water, alcohols, or moisture in the air to hydrofluoric acid and silicic acid, forming flammable and potentially explosive hydrogen gas. Attacks many metals in the presence of moisture.
Waste Disposal
For laboratory quantities, transfer into an evaporating dish containing sodium bicarbonate, spray with ammonia (6M NH4OH)/6 M-ammonium hydroxide/while stirring and then spread with crushed ice. Continue spraying with ammonia until the smoke of ammonium chloride partly subsides and add iced water while stirring. Neutralize and slowly transfer the mixture into a drain with running water.
Properties of Silicon Tetrafluoride
| Melting point: | -90°C |
| Boiling point: | -86 °C |
| Density | 1.66 |
| vapor pressure | 3517.191kPa at 25℃ |
| solubility | reacts with H2O |
| form | colorless gas |
| Specific Gravity | 1.66 |
| color | colorless |
| Water Solubility | hydrolyzed by H2O [AIR87] |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| Merck | 13,8575 |
| CAS DataBase Reference | 7783-61-1(CAS DataBase Reference) |
| EPA Substance Registry System | Silane, tetrafluoro- (7783-61-1) |
Safety information for Silicon Tetrafluoride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P350:IF ON SKIN: Gently wash with plenty of soap and water. |
Computed Descriptors for Silicon Tetrafluoride
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1