CESIUM DODECAHYDRODODECABORATE
- CAS NO.:12008-75-2
- Empirical Formula: B12Cs2H12
- Molecular Weight: 407.64
- MDL number: MFCD00273353
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
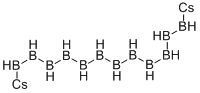
What is CESIUM DODECAHYDRODODECABORATE?
Description
Cesium dodecaborate is one of many known salts of polyborane anions. The dodecaborate anion has the shape of a regular octahedron, one of the five platonic solids. Dodecaborate is isoelectronic with dodecahedrane, the Molecule of the Week for February 22, 2021.
In 1960, Anthony R. Pitochelli and M. Frederick Hawthorne1 at Rohm and Haas’s Redstone Arsenal Research Division (Huntsville, AL) prepared small amounts of some dodecaborate salts. The salts contained trimethylammonium, potassium, and triphenylmethylphosphonium cations.
Later in the decade, Henry C. Miller and Earl L. Muetterties at Du Pont (Wilmington, DE), developed a synthesis of the cesium salt. As described in the 1967 book Borane Anions2, the synthesis begins with the reaction of sodium borohydride (NaBH4) with boron trifluoride (BF3) to produce the triborate NaB3H8. The triborate is then pyrolyzed to give a mixture that includes sodium dodecaborate. The desired anion is obtained by precipitating it as the cesium salt.
Thomas Schlied and co-workers at the University of Stuttgart (Germany) published the crystal structure of cesium dodecaborate in 2000. The crystals are colorless, face-rich cubic with B–B distances of 178 pm and B–H distances of 112 pm. Each cesium ion is in contact with 12 hydrogen atoms, each at a distance of 313 pm.
In recent years, scientists have been exploring practical applications of dodecaborate salts, such as in battery electrodes, catalysis, and sensors. In particular, a research team led by Haibo Zhang and Xiaohai Zhou at the University of Wuhan (China) have used cesium dodecaborate to prepare complex catalysts.
In 2018, the researchers reported the preparation of a class of core–shell magnetic gold nanocomposites with “raspberry-like” structures. Cesium dodecaborate played a dual role in preparing highly monodispersed gold nanoparticles (AuNPs) and immobilizing them on the surface of a γCD@Fe3O4 substrate3. The resulting AuNPs@Fe3O4 composites made highly active and recyclable catalysts for the selective reduction of nitroaromatic compounds to the corresponding anilines.
Two years later, the group showed that cubic platinum nanoparticles capped with cesium dodecaborate catalyzes the direct oxidation of methane by hydrogen peroxide and oxygen to ethanol under mild (50 °C) conditions. The optimized reaction gave >97% conversion to ethanol.
1. Hawthorne, a giant in the field of boron chemistry, passed away last month at age 92. Nicknamed “Mr. Boron” by his associates, he was awarded the prestigious ACS Priestly Medal in 2009.
2. Hawthorne, Miller, and Muetterties, among others, were coauthors.
3. γCD@Fe3O4 is a γ-cyclodextrin–hematite complex.
Properties of CESIUM DODECAHYDRODODECABORATE
| Melting point: | >650 °c |
| solubility | slight |
| form | Powder |
| appearance | white crystals or powder |
| color | white |
| Stability: | hygroscopic |
| EPA Substance Registry System | Dodecaborate(2-), dodecahydro-, dicesium (12008-75-2) |
Safety information for CESIUM DODECAHYDRODODECABORATE
Computed Descriptors for CESIUM DODECAHYDRODODECABORATE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

![2,2,6,6-TETRAMETHYL-3,5-HEPTANEDIONATO CESIUM [CS(TMHD)]](https://img.chemicalbook.in/CAS/GIF/61346-75-6.gif)
You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
