Cesium chloride
Synonym(s):Caesium chloride;Cesium chloride;Cesium chloride solution;Cesium Chloride, Molecular Biology Grade - CAS 7647-17-8 - Calbiochem;Cesium monochloride
- CAS NO.:7647-17-8
- Empirical Formula: ClCs
- Molecular Weight: 168.36
- MDL number: MFCD00010955
- EINECS: 231-600-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
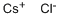
What is Cesium chloride?
Chemical properties
Cesium chloride (CsCl) is an inorganic, colorless, hygroscopic crystalline powder. It has a large mass and is highly soluble in water (1865 g/L). Due to its hygroscopic characteristic, when put in water, it forms a dense solute that is not very viscous. Therefore, it is a good material for equilibrium gradient differential centrifugation where the separation of the particles is size and density dependent.
Cesium Chloride is a type of unit cell that is commonly mistaken as Body-Centered Cubic. This misconception is easy to make, since there is a center atom in the unit cell, but CsCl is really a non-closed packed structure type.
History
Someone has found that CsCl is described as showing a blue glow in the dark. It is interpreted that light can be caused by the collision of subatomic particles that escape from the nucleus of cesium 137 and collide with electrons located on the periphery of the atom, so the energy of the electrons increases, to return to the previous equilibrium state, the electrons release the excess of energy emitting light.
It is also explained that because it is a highly active light source used in cancer treatment, blue light can be seen by the naked eye. The blue light is due to fluorescent UV dominant optical emission caused by beta, gamma, and Ba X-ray emissions from within the same excited 137-Cs atoms by a previously unknown atomic phenomenon, now known as Padmanabha Rao effect. But looking at Wikipedia, the IAEA report does not explain whether the blue light emitted by 137-c is fluorescence or Cerenkov radiation.
The Uses of Cesium chloride
Cesium chloride is used in radio and television vacuum tubes. It also is used in ultracentrifuge separations; x-ray fluorescent screens; as radiogrpahic contrast medium, and to prepare cesium and other cesium salts.
Used for the preparation of electrically conducting glasses.
Used to make solutions for the separation of RNA from DNA by density gradient centrifugation.
For molecular genetic applications used in preparing nucleic acids for subsequent hybridization or cloning experiments.
Preparation
Cesium chloride (CsCl) is produced by the reaction of cesium metal with chlorine gas (Ca+ + Cl- → CsCl). It is also used in the beer brewing industry, to coat fluorescent screens, and to improve the taste of mineral water.
The Uses of Cesium chloride
Cesium chloride has been used in density gradient centrifugation and for fractionation of nucleic acids, ribosomal subunits, proteins, glycoproteins, and viruses. Cesium chloride can also be used as a raw material to prepare other cesium compounds, such as cesium chloride reacts with nitric acid and oxalate to prepare cesium carbonate. It can be applied to the discharge tube (positive ion provided by the filament surface) fluorescent screen and used as a contrast agent. It effectively prevents activation of caspase-3 and neuronal apoptosis in serum- and potassium-deprived cerebellar granule neurons by inactivating GSK-3β.
What are the applications of Application
Cesium Chloride is a useful compound for density gradient centrifugation and various molecular biology applications.
Definition
ChEBI: Caesium chloride is the inorganic chloride salt of caesium; each caesium ion is coordinated by eight chlorine ions. It has a role as a phase-transfer catalyst and a vasoconstrictor agent. It is an inorganic chloride and an inorganic caesium salt.
What are the applications of Application
Cesium chloride has been used in the generation of discontinuous gradient for the purification of Cryptosporidium oocysts and as a component of acetamide medium for fungal selection.
Cesium chloride may be employed in gel-electrophoresis and RNA purification studies. It may be employed for the isolation of bacterial plasmid from Agrobacterium spp.
brand name
Cescan-131 (Abbott).
General Description
Cesium chloride is a cesium halide. Cesium halides can be prepared by reacting cesium carbonate with corresponding hydrohalic acids. Cesium chloride ultracentrifugation method has been reported for the extraction of RNA from cellular fractions.
Health Hazard
Whereas conventional caesium chloride has a rather low toxicity to humans and animals, the radioactive form easily contaminates the environment due to the high solubility of CsCl in water. Spread of 137CsCl powder from a 93-gram container in 1987 in Goiania, Brazil, resulted in one of the worst-ever radiation spill accidents killing four and directly affecting 249 people.
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
Oral intake of cesium chloride is known to increase the pH in cancer cells. Mild toxicity of cesium chloride might cause hypotension, gastrointestinal distress, numbness and syncope. It also leads to severe hypokalemia, hypomagnesemia, acute heart attack and polymorphic ventricular tachycardia in episodes.
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. Experimental reproductive effects. Mutation data reported. Reacts violently with BF3. See also CESIUM. When heated to decomposition it emits toxic fumes of Cl-.
Purification Methods
It is soluble in H2O but can be purified by crystallisation from H2O [solublity in g percent: 162.3(0.7o), 182.2(16.2o) and 290(at bp 119.4o)] and dried in high a vacuum. It is soluble in EtOH and is deliquescent; keep it in a tightly closed container. [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 951-955 1963.] For further purification of CsCl, a concentrated aqueous solution of the practically pure reagent is treated with an equivalent weight of I2 and Cl2 is bubbled into the solution until preciptation of CsCl2I is complete. Recrystallisation yields a salt which is free from other alkali metals. It is then decomposed to pure CsCl on heating. [Harned & Schupp J Am Chem Soc 52 3886 1930.] It can also be recrystallised from acetone/water, or from water (0.5mL/g) by cooling in a CaCl2/ice bath. Dry it at 78o under vacuum.
Properties of Cesium chloride
| Melting point: | 645 °C (lit.) |
| Boiling point: | 1290 °C |
| Density | 3.988 g/cm3 |
| vapor density | 5.8 (vs air) |
| refractive index | 1.6418 |
| Flash point: | 1303°C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 3 M at 20 °C, clear, colorless |
| form | beads |
| color | White |
| Specific Gravity | 3.988 |
| PH Range | 6.0 - 7.5 |
| PH | 5.0-7.5 (25℃, 3M in H2O) |
| Odor | Odorless |
| Water Solubility | 1860 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.02 λ: 280 nm Amax: 0.02 |
| Merck | 14,2011 |
| Stability: | Stable. Deliquescent. Incompatible with strong oxidizing agents, strong acids. Protect from moisture. |
| CAS DataBase Reference | 7647-17-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Cesium chloride(7647-17-8) |
| EPA Substance Registry System | Cesium chloride (CsCl) (7647-17-8) |
Safety information for Cesium chloride
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Cesium chloride
| InChIKey | AIYUHDOJVYHVIT-UHFFFAOYSA-M |
Cesium chloride manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 CESIUM CHLORIDE 99%View Details
CESIUM CHLORIDE 99%View Details -
 Cesium chloride CAS 7647-17-8View Details
Cesium chloride CAS 7647-17-8View Details
7647-17-8 -
 Cesium chloride CAS 7647-17-8View Details
Cesium chloride CAS 7647-17-8View Details
7647-17-8 -
 Cesium chloride, Ultra dry CAS 7647-17-8View Details
Cesium chloride, Ultra dry CAS 7647-17-8View Details
7647-17-8 -
 Cesium chloride, Ultra dry CAS 7647-17-8View Details
Cesium chloride, Ultra dry CAS 7647-17-8View Details
7647-17-8 -
 Cesium chloride CAS 7647-17-8View Details
Cesium chloride CAS 7647-17-8View Details
7647-17-8 -
 Cesium chloride CAS 7647-17-8View Details
Cesium chloride CAS 7647-17-8View Details
7647-17-8 -
 Cesium chloride CAS 7647-17-8View Details
Cesium chloride CAS 7647-17-8View Details
7647-17-8
