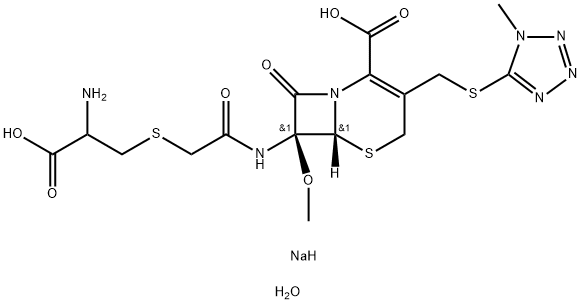
Cefuroxime sodium
Synonym(s):Cefuroxime sodium salt
- CAS NO.:56238-63-2
- Empirical Formula: C16H15N4O8S.Na
- Molecular Weight: 446.37
- MDL number: MFCD09878727
- EINECS: 260-073-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04

What is Cefuroxime sodium ?
Chemical properties
White Crystalline Solid
Originator
Ultroxim,Duncan,Italy,1978
The Uses of Cefuroxime sodium
Cefuroxime Sodium Salt is an antibacterial.
The Uses of Cefuroxime sodium
Cephalosporin antibacterial.
What are the applications of Application
Cefuroxime Sodium Salt is an antibacterial compound
Definition
ChEBI: Cefuroxime sodium is an organic molecular entity.
Manufacturing Process
A stirred mixture of N,N-dimethylacetamide (75 ml), acetonitrile (75 ml),
triethylamine (42 ml, 0.3 mol) and (6R,7R)-7-amino-3-carbamoyloxy-methylceph-3-em-4-carboxylic acid was immersed in an ice-bath and water
(10 ml) was added. The mixture was stirred at 0°C to 2°C for 45 minutes, the
solid slowly dissolving to give a yellow solution.
Meanwhile a stirred suspension of phosphorus pentachloride (14.99 g, 0.072
mol) in dry dichloromethane (150 ml) was cooled to 0°C, and N,N-dimethylacetamide (27.5 ml) was added. The resulting solution was recooled
to -10°C and 2-(fur-2-yl)-2-methoxyiminoacetic acid (synisomer) (12.17 g,
0.072 mol) was added. The mixture was stirred at -10°C for 15 minutes and
crushed ice (35 g) was added. The mixture was stirred at 0°C for 10minutes,
where after the lower dichloromethane phase was added over 10 minutes to
the cephalosporin solution prepared above, cooled to -10°C so that the
reaction temperature rose steadily to 0°C. The mixture was stirred at 0°C to
2°C for 1 hour, where after the cooling bath was removed and the reaction
temperature allowed to rise to 20°C over 1 hour. The reaction mixture was
then added slowly to 2 N hydrochloric acid (100 ml) diluted with cold water
(1.15 l) at 5°C. The pH of the two phase mixture was adjusted to below 2
with 2 N hydrochloric acid (10 ml), and the mixture was stirred and recooled
to 5°C. The solid which precipitated was filtered, washed with
dichloromethane (100 ml) and water (250 ml), and dried in vacuo at 40°C
overnight to give the title compound (22.04 g, 86.6%).
brand name
Kefurox (Lilly); Zinacef (GlaxoSmithKline).
Therapeutic Function
Antibiotic
Clinical Use
Cefuroxime (Zinacef) is the first of a series of α-methoximinoacyl–substituted cephalosporins that constitute most of thethird-generation agents available for clinical use. A syn alkoximinosubstituent is associated with β-lactamase stability in these cephalosporins.78 Cefuroxime is classified as a secondgenerationcephalosporin because its spectrum of antibacterialactivity more closely resembles that of cefamandole. It is,however, active against β-lactamase–producing strains thatare resistant to cefamandole, such as E. coli, K. pneumoniae,N. gonorrhoeae, and H. influenzae. Other important Gramnegativepathogens, such as Serratia, indole-positive Proteusspp., P. aeruginosa, and B. fragilis, are resistant.Cefuroxime is distributed throughout the body. It penetratesinflamed meninges in high enough concentrations tobe effective in meningitis caused by susceptible organisms.Three-times-daily dosing is required to maintain effectiveplasma levels for most sensitive organisms, such asNeisseria meningitidis, Streptococcus pneumoniae, and H.influenzae. It has a plasma half-life of 1.4 hours.
Properties of Cefuroxime sodium
| Melting point: | 240-245°C(dec) |
| alpha | D20 +60° (c = 0.91 in water) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Freely soluble in water, very slightly soluble in ethanol (96 per cent). |
| form | neat |
| form | Solid |
| pka | pKa (water): 2.5; (DMF): 5.1(at 25℃) |
| color | White to Pale Beige |
| Water Solubility | Freely soluble in water |
| CAS DataBase Reference | 56238-63-2(CAS DataBase Reference) |
Safety information for Cefuroxime sodium
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for Cefuroxime sodium
| InChIKey | URDOHUPGIOGTKV-JTBFTWTJSA-M |
Cefuroxime sodium manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
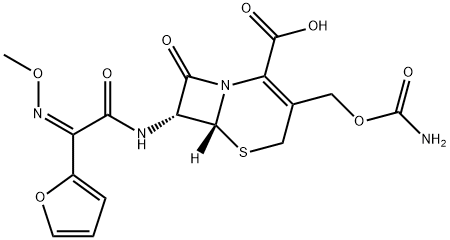

You may like
-
 Cefuroxime Sodium Sterile 99%View Details
Cefuroxime Sodium Sterile 99%View Details -
 Cefuroxime Sodium sterile 98%View Details
Cefuroxime Sodium sterile 98%View Details -
 Cefuroxime Sodium sterile 98%View Details
Cefuroxime Sodium sterile 98%View Details -
 Cefuroxime Sodium sterile 99%View Details
Cefuroxime Sodium sterile 99%View Details -
 Cefuroxime Sodium sterile 98%View Details
Cefuroxime Sodium sterile 98%View Details -
 Cefuroxime Sodium sterile 99%View Details
Cefuroxime Sodium sterile 99%View Details -
 Cefuroxime sodium 95% CAS 56238-63-2View Details
Cefuroxime sodium 95% CAS 56238-63-2View Details
56238-63-2 -
 Cefuroxime Sodium Salt CAS 56238-63-2View Details
Cefuroxime Sodium Salt CAS 56238-63-2View Details
56238-63-2
