Cefoxitin sodium
Synonym(s):Cefoxitin sodium salt
- CAS NO.:33564-30-6
- Empirical Formula: C16H18N3NaO7S2
- Molecular Weight: 451.44
- MDL number: MFCD00079042
- EINECS: 251-574-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
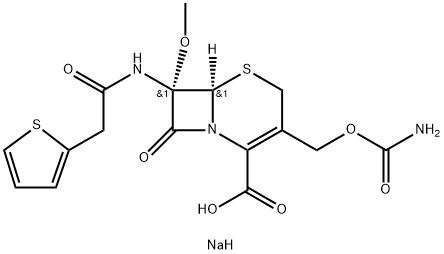
What is Cefoxitin sodium?
Description
Cefoxitin is a second generation cephalosporin antibiotic that has been used to treat a wide range of gram-
Chemical properties
Solid
Originator
Mefoxin,Merck Sharp and Dohme,US,1978
The Uses of Cefoxitin sodium
Cefoxitin sodium can be used as semi-synthetic antibiotic derived from Cephamycin C, possessing high resistance to β-lactamase inactivation.
The Uses of Cefoxitin sodium
An antibiotic derived from Cephamycin C.
The Uses of Cefoxitin sodium
A broad spectrum antibiotic
What are the applications of Application
Cefoxitin sodium salt is a semi-synthetic antibiotic derived from Cephamycin C and possesses a high resistance to Beta lactamase inactivation
Definition
ChEBI: Cefoxitin sodium is an organic molecular entity.
Manufacturing Process
Benzhydryl 3-carbamoyloxymethyl-7α-hydroxy-7β-(2-thienylacetamido)
decephalosporanate, 543 mg, is stirred in 15 ml dry DMSO. Sodium hydride,
24 mg (48 mg of a 50% suspension of NaH in mineral oi1, which has been
washed with hexane to remove the oil), is added. When hydrogen evolution
has ceased, 126 mg dimethyl sulfate is added. The solution is stirred for one
hour at room temperature, diluted with 100 ml benzene and washed six times
with water; the last wash is made to pH 8, if necessary, by adding sodium
bicarbonate. The solution is dried over MgSO4, filtered and evaporated,
leaving benzhydryl 3-carbamoyloxymethyl-7β-(2-thienylacetamido)-7α-
methoxydecephalosporanate, which may be purified if desired by
chromatography on silica gel, eluting with 25:1 chloroformethyl acetate.
Other methylating agents may be used in place of methyl sulfate, e.g., an
equimolar amount of methyl iodide, bromide or chloride, using the same
conditions, or methyl trifluoromethylsulfonate or trimethyloxonium
trinitrobenzenesulfonate. The solvent in the latter two reagents is dimethyl
ether-HMPA 1:1, using a reaction temperature of -20°C warming later to
25°C. In each instance, the benzhydryl 3-carbamoyloxymethyl-7β-(2-
thienylacetamido)-7α-methoxydecephalosporanate is obtained.
Benzhydryl 3-carbamoyloxymethyl-7β-(2-thienylacetamido)-7α-
methoxydecephalosporanate (300 mg) in 0.5 ml in anisole and 2.5 ml of
trifluoroacetic acid is reacted for 15 minutes at 10°C. The resulting mixture is evaporated at reduced pressure and flushed twice with anisole. The residue is
dissolved in methylene chloride and extracted with 5% sodium bicarbonate
solution. The aqueous solution is adjusted to pH 1.8 with 5% phosphoric acid
and extracted with ethyl acetate. The organic solution is dried and evaporated
to yield the pure 3-carbamoyloxymethyl-7α-methoxy-7β-(2-
thienylacetamido)decephalosporanic acid, MP 165°C to 167°C. This may then
be converted to the sodium salt.
brand name
Mefoxin (Merck).
Therapeutic Function
Antibiotic
Clinical Use
Cefoxitin (Mefoxin) is a semisynthetic derivative obtainedby modification of cephamycin C, a 7α-methoxy-substitutedcephalosporin isolated independently from variousStreptomyces by research groups in Japan and the UnitedStates. Although it is less potent than cephalothin againstGram-positive bacteria and cefamandole against most of theEnterobacteriaceae, cefoxitin is effective against certainstrains of Gram-negative bacilli (e.g., E. coli, K. pneumoniae,Providencia spp., S. marcescens, indole-positiveProteus spp., and Bacteroides spp.) that are resistant to thesecephalosporins. It is also effective against penicillin-resistantS. aureus and N. gonorrhoeae. The activity of cefoxitin and cephamycins, in general,against resistant bacterial strains is because of their resistanceto hydrolysis by β-lactamases conferred by the 7α-methoxylsubstituent. Cefoxitin is a potent competitive inhibitor ofmany β-lactamases. It is also a potent inducer of chromosomallymediated β-lactamases. The temptation to exploit the β-lactamase–inhibiting properties of cefoxitin by combining itwith β-lactamase–labile β-lactam antibiotics should be temperedby the possibility of antagonism. In fact, cefoxitin antagonizesthe action of cefamandole against E. cloacae andthat of carbenicillin against P. aeruginosa. Cefoxitin aloneis essentially ineffective against these organisms.
The pharmacokinetic properties of cefoxitin resemblethose of cefamandole. Because its half-life is relativelyshort, cefoxitin must be administered 3 or 4 times daily.Solutions of the sodium salt intended for parenteral administrationare stable for 24 hours at room temperature and 1week if refrigerated. 7α-Methoxyl substitution stabilizes, tosome extent, the β-lactam to alkaline hydrolysis.The principal role of cefoxitin in therapy seems to be forthe treatment of certain anaerobic and mixed aerobic–anaerobicinfections. It is also used to treat gonorrhea caused byβ-lactamase–producing strains. It is classified as a secondgenerationagent because of its spectrum of activity.
Veterinary Drugs and Treatments
In the United States, there are no cefoxitin products approved for veterinary species, but it has been used clinically in several species when an injectable second generation cephalosporin may be indicated.
References
[1]. miller ak, celozzi e, kong y, et al. cefoxitin, a semisynthetic cephamycin antibiotic: in vivo evaluation. antimicrob agents chemother. 1974 jan;5(1):33-7.
[2]. jacoby ga. ampc beta-lactamases. clin microbiol rev.2009 jan;22(1):161-82.
Properties of Cefoxitin sodium
| Melting point: | >160°C |
| Boiling point: | 843℃ |
| alpha | 25589nm +210° (c = 1 in methanol) |
| Flash point: | >110°(230°F) |
| storage temp. | 2-8°C |
| solubility | Very soluble in water, sparingly soluble in alcohol. |
| form | neat |
| form | Solid |
| color | White |
| Water Solubility | Soluble in water or methanol |
| CAS DataBase Reference | 33564-30-6(CAS DataBase Reference) |
Safety information for Cefoxitin sodium
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Cefoxitin sodium
| InChIKey | GNWUOVJNSFPWDD-XMZRARIVSA-M |
| SMILES | N([C@@]1(C(=O)N2C(=C(COC(=O)N)CS[C@]12[H])C(=O)O)OC)C(=O)CC1SC=CC=1.[NaH] |&1:1,14,r| |
Cefoxitin sodium manufacturer
Nectar Lifesciences Ltd
Aruvi Labs
JA Sterile Pvt Ltd
Anand Agencies
Aruvi Labs
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 33564-30-6 Cefoxitin 98%View Details
33564-30-6 Cefoxitin 98%View Details
33564-30-6 -
 Cefoxitin sodium sterile 98%View Details
Cefoxitin sodium sterile 98%View Details -
 Cefoxitin Sodium Salt (CTX) CAS 33564-30-6View Details
Cefoxitin Sodium Salt (CTX) CAS 33564-30-6View Details
33564-30-6 -
 Cefoxitin sodium 98% (HPLC) CAS 33564-30-6View Details
Cefoxitin sodium 98% (HPLC) CAS 33564-30-6View Details
33564-30-6 -
 Cefoxitin Sodium CAS 33564-30-6View Details
Cefoxitin Sodium CAS 33564-30-6View Details
33564-30-6 -
 Cefoxitin sodium sterile 98%View Details
Cefoxitin sodium sterile 98%View Details -
 Cefoxitin sodium CAS 33564-30-6View Details
Cefoxitin sodium CAS 33564-30-6View Details
33564-30-6 -
 Cefoxitin sodium salt CAS 33564-30-6View Details
Cefoxitin sodium salt CAS 33564-30-6View Details
33564-30-6