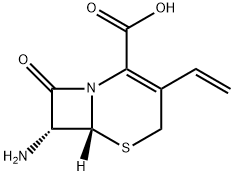
Ceftobiprole
- CAS NO.:209467-52-7
- Empirical Formula: C20H22N8O6S2
- Molecular Weight: 534.57
- MDL number: MFCD09954116
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
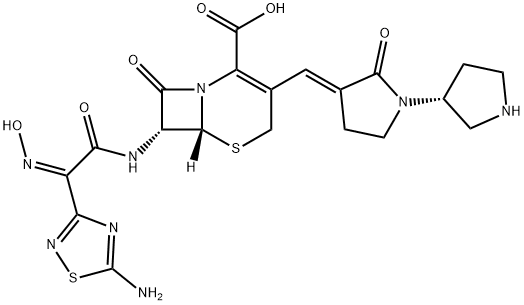
What is Ceftobiprole?
The Uses of Ceftobiprole
Broad spectrum antibiotic.
Background
Ceftobiprole is a cephalosporin antibiotic with activity against methicillin-resistant Staphylococcus aureus. It was discovered by Basilea Pharmaceutica and is being developed by Johnson & Johnson Pharmaceutical Research and Development. Ceftobiprole is the first cephalosporin to demonstrate clinical efficacy in patients with infections due to methicillin-resistant staphylococci and, if approved by regulatory authorities, is expected to be a useful addition to the armamentarium of agents for the treatment of complicated skin infections and pneumonia.
Indications
For the treatment of serious bacterial infections in hospitalised patients.
Definition
ChEBI: Ceftobiprole is a fifth-generation cephalosporin antibiotic having (E)-[(3'R)-2-oxo[1,3'-bipyrrolidin]-3-ylidene]methyl and [(2Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetyl]amino side groups located at positions 3 and 7 respectively; developed for the treatment of hospital-acquired pneumonia (HAP, excluding ventilator-associated pneumonia, VAP) and community-acquired pneumonia (CAP). It has a role as an antimicrobial agent. It is a cephalosporin and a member of thiadiazoles.
Antimicrobial activity
The most important property distinguishing it from older
cephalosporins is activity against methicillin-resistant staphylococci,
a property conferred by a high affinity for penicillinbinding
protein 2′ (2a). MICs for methicillin-resistant strains
are nevertheless somewhat higher than those seen with fully
susceptible strains. A similar situation exists with coagulasenegative
staphylococci and with Str. pneumoniae, for which
strains with reduced susceptibility to penicillin are less susceptible
than fully resistant strains, while remaining within
therapeutically achievable levels.
Otherwise activity approximates to that of cephalosporins
of group 4 . Activity against Ps. aeruginosa is modest
and much reduced against ceftazidime-resistant strains.
Acquired resistance
It is hydrolyzed by extended spectrum β-lactamases of enterobacteria , which are therefore resistant. The prospects for the emergence of resistance during extensive clinical use are presently unclear, though increased resistance in Staph. aureus appears to be difficult to induce under laboratory conditions.
Pharmacokinetics
Ceftobiprole, a cephalosporin antibiotic, is active against methicillin-resistant Staphylococcus aureus.
Pharmacokinetics
Cmax 500 mg (667 mg prodrug): c. 35 mg/L end infusion
intravenous (30-min infusion)
Plasma half-life: c. 3 h
Volume of distribution: 18.4 L
Plasma protein binding: 16%
The prodrug is rapidly hydrolyzed in plasma to release the
active form together with diacetyl (2,3-butanediol) and CO2.
Distribution approximates to the extracellular fluid volume in
adults. There is no accumulation on repeat dosing in subjects
with normal renal function.
It is chiefly excreted in urine by glomerular filtration. A urinary
concentration exceeding 1 g/L is achieved within the first
2 h of a 500 mg (active drug equivalent) dose and 80–90% of
active drug can be recovered within 24 h.
Clinical Use
Ceftobiprole can be used as Broad spectrum antibiotic and in complicated infections of skin and skin structures.
Side Effects
Limited studies have so far revealed no unexpected side effects. Nausea, vomiting and altered taste sensation appear to be the most common.
Metabolism
Not Available
Properties of Ceftobiprole
| Density | 2.00±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C,unstable in solution, ready to use. |
| solubility | DMSO : 5 mg/mL (9.35 mM) |
| form | Solid |
| pka | 2.46±0.50(Predicted) |
| color | Light yellow to yellow |
Safety information for Ceftobiprole
Computed Descriptors for Ceftobiprole
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



![(6R,7R)-7-Amino-8-oxo-3-(1-propenyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid](https://img.chemicalbook.in/CAS/GIF/120709-09-3.gif)
You may like
-
 Ceftobiprole CAS 209467-52-7View Details
Ceftobiprole CAS 209467-52-7View Details
209467-52-7 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1