Unii-p9vxv1408y
- CAS NO.:866021-48-9
- Molecular Weight: 0
- MDL number: MFCD19443689
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-13 18:13:23
What is Unii-p9vxv1408y?
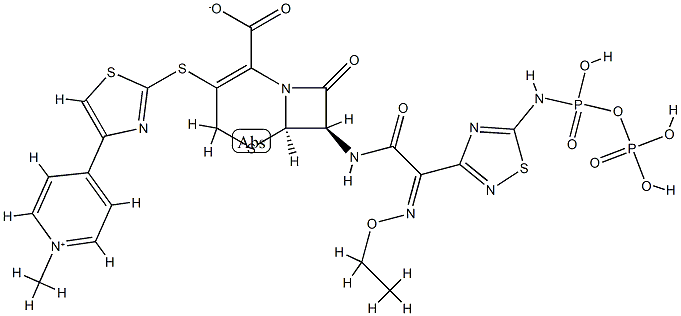
Description
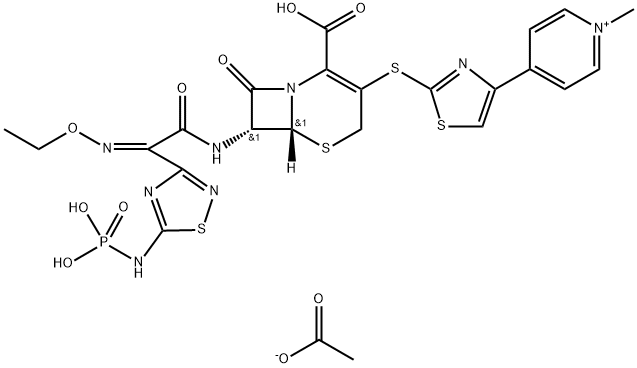
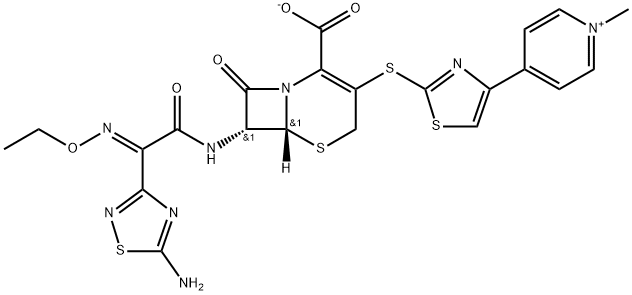
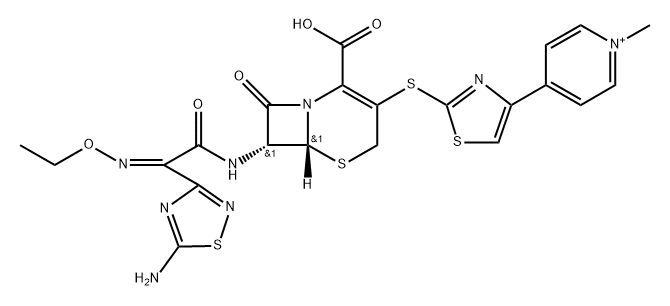
Ceftaroline (trade names Teflaro and Zinforo) is a cephalosporin-type antibiotic that is active against methicillin-resistant?Staphylococcus aureus?(MRSA). It was first approved by the US Food and Drug Administration in 2010 for treating community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections, including MRSA.
By 2014, however, MRSA had mutated into strains that are resistant to ceftaroline. S. Mobashery at the University of Notre Dame, J. A. Hermoso at the Spanish National Research Council, and colleagues showed that the cause of the resistance is a previously unknown mechanism that?prevents the antibiotic from binding to a bacterial protein. The researchers believe that this mechanism may also exist in other antibiotic-resistant organisms.
Definition
ChEBI: Ceftaroline fosamil acetate monohydrate is a hydrate that is the monohydrate form of ceftaroline fosamil acetate. A prodrug for ceftaroline, used for the treatment of adults with acute bacterial skin and skin structure infections. It has a role as an antimicrobial agent, an antibacterial drug and a prodrug. It contains a ceftaroline fosamil acetate.
Antimicrobial activity
A semisynthetic cephalosporin formulated as the water-soluble fosamil acetate prodrug for intravenous administration. Its properties are similar to those of ceftobiprole, with which it shares an enhanced affinity for penicillin-binding protein 2′ (2a) of methicillin-resistant Staph. aureus. It is hydrolyzed by extended-spectrum β-lactamases and is not active against Amp-C derepressed strains of Gram-negative bacilli.
Pharmacokinetics
Cmax 600 mg intravenous (1-h infusion): 19 mg/L end infusion
Plasma half-life: 2.6 h
Volume of distribution: 0.37 L/kg
Plasma protein binding :<20%
Like ceftobiprole, ceftaroline fosamil is rapidly hydrolyzed in
plasma after intravenous infusion and excreted principally in urine.
In preliminary clinical studies it appears to be well tolerated.
Safety information for Unii-p9vxv1408y
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8