Cefotetan disodium
- CAS NO.:74356-00-6
- Empirical Formula: C17H18N7NaO8S4
- Molecular Weight: 599.6
- MDL number: MFCD00941449
- EINECS: 277-834-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
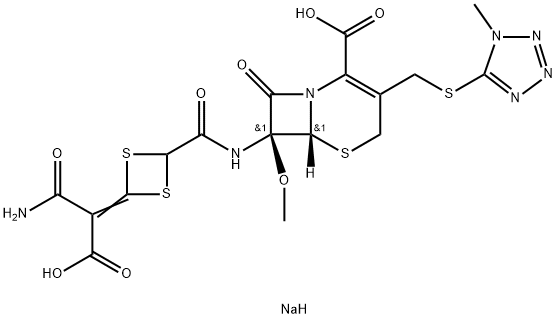
What is Cefotetan disodium?
Description
Cefotetan disodium is a β-lactamase resistant, second generation cephalosporin with a serum half-life of about three hours, permitting twice-daily dosing.
Originator
Yamanouchi (Japan)
The Uses of Cefotetan disodium
Antibacterial.
The Uses of Cefotetan disodium
Cefotetan Disodium is a related compound of Cefotetan (C242970), an antibiotic related to Cephalosporin that is used to treat bacterial infections.
What are the applications of Application
Cefotetan disodium is a cephamycin antibiotic
Definition
ChEBI: The disodium salt of cefotetan.
brand name
Cefotan (AstraZeneca);YAMATETAN.
Clinical Use
Cefotetan (Cefotan) is a third-generation cephalosporin thatis structurally similar to cefoxitin. Like cefoxitin, cefotetanis resistant to destruction by β-lactamases. It is also a competitiveinhibitor of many β-lactamases and causes transient inactivation of some of these enzymes. Cefotetan is reportedto synergize with β-lactamase–sensitive β-lactams but, unlikecefoxitin, does not appear to cause antagonism.
The antibacterial spectrum of cefotetan closely resemblesthat of cefoxitin. It is, however, generally more activeagainst S. aureus, and members of the Enterobacteriaceaefamily sensitive to both agents. It also exhibits excellentpotency against H. influenzae and N. gonorrhoeae, includingβ-lactamase–producing strains. Cefotetan is slightly lessactive than cefoxitin against B. fragilis and other anaerobes.Enterobacter spp. are generally resistant to cefotetan, andthe drug is without effect against Pseudomonas spp.Cefotetan has a relatively long half-life of about 3.5hours. It is administered on a twice-daily dosing schedule. Itis excreted largely unchanged in the urine. Aqueoussolutions for parenteral administration maintain potency for24 hours at 25°C. Refrigerated solutions are stable for 4 days.
Cefotetan contains the MTT group that has been associatedwith hypoprothrombinemia and alcohol intolerance.Another cephalosporin that lacks these properties should beselected for patients at risk for severe bleeding or alcoholism.
Veterinary Drugs and Treatments
Cefotetan may be a reasonable choice for treating serious infections caused by susceptible bacteria, including E. coli or anaerobes. It appears to be well tolerated in small animals and may be given less frequently than cefoxitin.
Properties of Cefotetan disodium
| Boiling point: | 798℃ |
| RTECS | XI0330600 |
| storage temp. | 2-8°C |
| solubility | Freely soluble in aqueous solution |
| form | powder |
Safety information for Cefotetan disodium
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P284:Wear respiratory protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. |
Computed Descriptors for Cefotetan disodium
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE 4-Bromopyrazole 5,6-Dimethoxyindanone Tert-butyl bis(2-chloroethyl)carbamate (2-Hydroxyphenyl)acetonitrile 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Cefotetan disodium 95.00% CAS 74356-00-6View Details
Cefotetan disodium 95.00% CAS 74356-00-6View Details
74356-00-6 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1