Ceforanide
- CAS NO.:60925-61-3
- Empirical Formula: C20H21N7O6S2
- Molecular Weight: 519.55
- MDL number: MFCD00210833
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
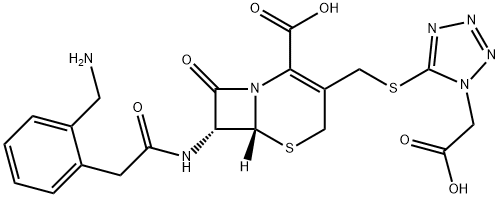
What is Ceforanide?
Absorption
Rapidly absorbed following intramuscular injection.
Toxicity
Adverse effects following overdosage include nausea, vomiting, epigastric distress, diarrhea, and convulsions.
Description
Ceforanide is a second generation cephalosporin with good β-lactamase resistance. It has a serum half-life of about three hours, allowing twice-daily dosing.
Originator
Bristol (USA)
The Uses of Ceforanide
Ceforanide is a cephalosporin based antibiotic used in the sterilization in various medical procedures.
The Uses of Ceforanide
Cephalosporin antibiotic
Background
Ceforanide is administered parenterally. It has a longer elimination half-life than any currently available cephalosporin. Its activity is very similar to that of cefamandole, another second-generation cephalosporin antibiotic, except that ceforanide is less active against most gram-positive organisms. Many coliforms, including Escherichia coli, Klebsiella, Enterobacter, and Proteus, are susceptible to ceforanide, as are most strains of Salmonella, Shigella, Hemophilus, Citrobacter and Arizona species.
Indications
For the treatment of infections caused by susceptible organisms.
Definition
ChEBI: A second-generation cephalosporin antibiotic with {[1-(carboxymethyl)-1H-tetrazol-5-yl]sulfanyl}methyl and 2-(aminomethyl)phenylacetamido groups at positions 3 and 7, respectively, of the cephem skeleton. It is effective against many col forms, including Escherichia coli, Klebsiella, Enterobacter and Proteus, and most strains of Salmonella, Shigella, Hemophilus, Citrobacter and Ari ona species.
brand name
Precef (Apothecon).
Antimicrobial activity
A semisynthetic parenteral cephalosporin with activity broadly
similar to that of cefalotin. Its activity in vitro is significantly
reduced in the presence of serum. A 1 g intravenous dose achieves a concentration of c. 135 mg/L at the end of infusion.
The response after 0.25, 0.5 and 1 g intravenous doses
is essentially linear. A 1 g intramuscular dose produces mean
peak values of around 70 mg/L. Plasma protein binding is
around 85%.
It is almost entirely eliminated in the urine with a halflife
of about 2.5 h, 80–95% being recovered in the first 12 h.
The half-life is inversely related to renal function, rising
to around 20 h when the creatinine clearance falls below
5 mL/min. About half the dose is removed by hemodialysis
over 6 h.
It is generally well tolerated; phlebitis and pain at the site
of injection are reported in some patients with occasional
transient neutropenia and increased transaminase levels.
It has been used principally for the treatment of infections
due to Gram-positive cocci, including staphylococcal and
streptococcal soft-tissue infections, but is no longer widely
available.
Pharmacokinetics
Ceforanide is a semisynthetic second-generation cephalosporin. The cephalosporins are bactericidal drugs with both gram-positive and gram-negative activity. They inhibit bacterial cell wall synthesis in a way similar to the penicillins.
Metabolism
The major drug elimination route was urinary excretion with 85% of the dose being excreted unchanged in the urine within 12 hr, and no metabolites with antibiotic activity were observed in urine.
Properties of Ceforanide
| Melting point: | >150° (dec) |
| Density | 1.79±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | Aqueous Base (Slightly), DMSO (Slightly, Heated), Methanol (Slightly) |
| form | Solid |
| pka | 2.52±0.10(Predicted) |
| color | White to Pale Brown |
| Stability: | Hygroscopic |
Safety information for Ceforanide
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P342+P311:IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. |
Computed Descriptors for Ceforanide
| InChIKey | SLAYUXIURFNXPG-CRAIPNDOSA-N |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran




You may like
-
 Ceforanide 98% CAS 60925-61-3View Details
Ceforanide 98% CAS 60925-61-3View Details
60925-61-3 -
 Ceforanide 96% CAS 60925-61-3View Details
Ceforanide 96% CAS 60925-61-3View Details
60925-61-3 -
 Ceforanide CAS 60925-61-3View Details
Ceforanide CAS 60925-61-3View Details
60925-61-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4