Cefatrizine
- CAS NO.:51627-14-6
- Empirical Formula: C18H18N6O5S2
- Molecular Weight: 462.5
- MDL number: MFCD01940014
- EINECS: 257-324-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-21 10:59:17
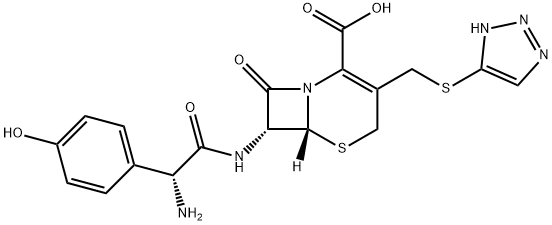
What is Cefatrizine?
Description
Cefatrizine was synthesized by BristolMyers Co. and Smith Klein & French Laboratories in 1974. It shows two to four times higher activity against gram-positive and four to eight times higher activity against gram-negative bacteria than cephalexin. Cefatrizine also shows excellent oral absorption, and its in vivo activity is 30 to 500 times higher than that of cephalexin.
Originator
Bricef,Bristol Banyu,Japan,1980
The Uses of Cefatrizine
Cefatrizine is a new orally administered cephalosporin with excellent activity against gram-positive cocci, inhibiting all except enterococci at minimal inhibitory concentrations below 1 μg/ml.
The Uses of Cefatrizine
Antidepressant, Selective serotonin reuptake inhibitor
Definition
ChEBI: A cephalosporin compound having (1H-1,2,3-triazol-4-ylsulfanyl)methyl and [(2R)-2-amino-2-(4-hydroxyphenyl)]acetamido side-groups. An antibacterial drug first prepared in the 1970s, it has more recently been found to be n inhibitor of eukaryotic elongation factor-2 kinase (eEF2K), which is known to regulate apoptosis, autophagy and ER stress in many types of human cancers.
Manufacturing Process
A total 6.5 g (1 1.55 mmol) of 7-[D-α-t-butoxycarbonylamino-α-(p-hydroxyphenyl)acetamido]-3-(1,2,3-triazol-5-ylthiomethyl)-3-cephem-4- carboxylic acid was dissolved in 175 ml (98 to 100% formic acid under anhydrous conditions. The mixture was stirred at room temperature for 2.5 hours. Part of the solution, 125 ml, was evaporated under reduced pressure to an amber oil. The oil was then azeotroped 3 times with 70 ml of toluene under reduced pressure. The residue was suspended in an 80:20 H2O-CH3OH solution (700 ml) and stirred for 0.5 hour until most of the solid dissolved, then filtered. The filtration was treated with 1.59 of (Darko) charcoal for about 20 minutes. The charcoal was filtered off through a Celite pad. The solution was then freeze-dried in 9 separate 100 ml round bottom flasks. The freeze-dried material weighed 2.415 g. It was recrystallized in batches of 0.200 g as described above to yield a total of 0.923 g 7-[D-α-amino-α-(p-hydroxyphenyl) acetamidol-3-(1,2,3-triazol-5-ylthiomethyl)-3-cephem-4-carboxylic acid. NMR was consistent, indicating the presence of 0.33 mol of CH3OH.
Therapeutic Function
Antibiotic
Antimicrobial activity
A semisynthetic cephalosporin formulated for oral use. The
spectrum is similar to that of cefalexin but it is more active
against H. influenzae. Wide strain variations in
susceptibility have been reported.
It is only partially absorbed when given by mouth. A
500 mg oral dose achieves a concentration of c. 6 mg/L after
1–2 h. The normal half-life of 2.5 h is extended to 5.5 h
in end-stage renal failure. Distribution resembles that of
cefalexin. It crosses the placenta readily. Plasma protein
binding is 40–60%.
Urinary recovery in 6 h is 35% of an oral dose, producing
urinary levels of 50–1500 mg/L. It is presumed that the
remainder is metabolized, but no metabolites have been
identified.
Apart from some mild diarrhea, it is well tolerated. It is
available in Japan.
Safety Profile
Moderately toxic byintraperitoneal and subcutaneous routes. An experimentalteratogen. Other experimental reproductive effects. Whenheated to decomposition it emits very highly toxic fumesof NOx and SOx.
Properties of Cefatrizine
| Boiling point: | 948.1±65.0 °C(Predicted) |
| Density | 1.3806 (rough estimate) |
| refractive index | 1.7000 (estimate) |
| storage temp. | Store at -20°C |
| solubility | DMSO: 95 mg/mL (201.02 mM);Ethanol: 23 mg/mL (48.67 mM) |
| pka | 2?+-.0.50(Predicted) |
| Water Solubility | Water: 95 mg/mL (201.02 mM) |
| CAS DataBase Reference | 51627-14-6(CAS DataBase Reference) |
Safety information for Cefatrizine
Computed Descriptors for Cefatrizine
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

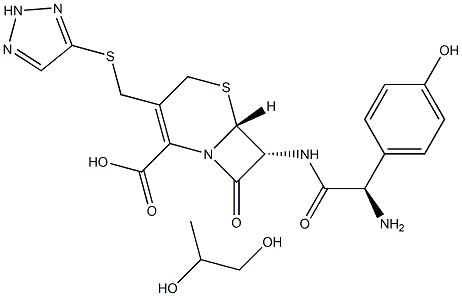
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
