Carprofen
Synonym(s):6-Chloro-α-methyl-9H-carbazole-2-acetic acid;CAR;Carprofen
- CAS NO.:53716-49-7
- Empirical Formula: C15H12ClNO2
- Molecular Weight: 273.71
- MDL number: MFCD00079028
- EINECS: 258-712-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
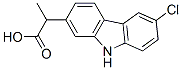
What is Carprofen?
Absorption
Rapidly and nearly completely absorbed (more than 90% bioavailable) when administered orally.
Toxicity
Symptoms of NSAID overdose include dizziness and nystagmus. Oral LD50 in mouse and rat is 282 mg/kg and 149 mg/kg, respectively.
Description
Carprofen is a non-steroidal anti-inflammatory drug (NSAID) commonly used in animals to combat pain and inflammation, particularly as associated with osteoarthritis. Like many NSAIDs, carprofen inhibits both cyclooxygenases COX-1 and COX-2 (IC50s = 22.3 and 3.9 μM, respectively). It also inhibits fatty acid amide hydrolase (IC50 = 74 μM), blocking the metabolism of the cannabinoid receptor ligand, arachidonoyl ethanolamide .
Chemical properties
Off-White Crystalline Solid
Originator
Imadyl,Roche,Switz.,1981
History
Carprofen has strong anti-inflammatory and analgesic activity. It has been used in human medicine for more than 10 years at doses of 150 to 600 mg per day. In human clinical trials, Carprofen was generally well tolerated. Most adverse reactions were transient and mild, such as gastrointestinal discomfort or pain and nausea. The incidence of side effects in humans is similar to that of aspirin and other NSAIDs. However, for commercial reasons, carprofen has been withdrawn from the market and is no longer available for human use.
The Uses of Carprofen
antiinflammatory, analgesic
The Uses of Carprofen
Carprofen is a non-steroidal anti-inflammatory drug (NSAID) commonly used in animals to combat pain and inflammation, particularly as associated with osteoarthritis. Like many NSAIDs, carprofen inhibits both cyclooxygenases COX-1 and COX-2 (IC50s = 22.3 and 3.9 μM, respectively). It also inhibits fatty acid amide hydrolase (IC50 = 74 μM), blocking the metabolism of the cannabinoid receptor ligand, arachidonoyl ethanolamide .
The Uses of Carprofen
Carprofen is a non steroidal anti-inflammatory that is used by veterinarians for the relief of arthritic systems in dogs. It can be used for joint pain or post operative inflammation. Carprofen, is al so used for the relief from pain and inflammation associated with osteoarthritis, hip dysplasia and other joint issues.
Indications
For use as a pain reliever in the treatment of joint pain and post-surgical pain.
Background
Carprofen is a non-steroidal anti-inflammatory drug (NSAID) that is used by veterinarians as a supportive treatment for the relief of arthritic symptoms in geriatric dogs. Carprofen was previously used in human medicine for over 10 years (1985-1995). It was generally well tolerated, with the majority of adverse effects being mild, such as gastro-intestinal pain and nausea, similar to those recorded with aspirin and other non-steroidal anti-inflammatory drugs. It is no longer marketed for human usage, after being withdrawn on commercial grounds.
What are the applications of Application
Carprofen is an antiinflammatory inhibitor of COX-2
Definition
ChEBI: Propanoic acid in which one of the methylene hydrogens is substituted by a 6-chloro-9H-carbazol-2-yl group. A non-steroidal anti-inflammatory drug, it is no longer used in human medicine but is still used for treatment of arthritis in elderly dogs.
Manufacturing Process
A mixture of 34.9 g of 6-chloro-α-methyl-1,2,3,4-tetrahydrocarbazole-2-acetic
acid ethyl ester (mixture of diastereomers), 350 ml CP xylene and 56.0 g of
p-chloranil was stirred and heated under an atmosphere of dry nitrogen. The
reaction flask was wrapped in aluminum foil in order to keep out any
extraneous light. After the reaction mixture had stirred at reflux temperature
for 6 hours, heating and stirring were stopped and the reaction mixture was
left overnight at room temperature. The supernatant liquid was decanted through a filter. The residue was triturated with 100 ml of warm benzene and
the supernatant liquid was decanted through a filter. This process was
repeated three more times. Ether (300 ml) was added to the combined
filtrates. The solution was extracted with cold 2 N sodium hydroxide (3 x 100
ml), washed by extraction with water until neutral and dried over anhydrous
magnesium sulfate. Following filtration of the desiccant and evaporation of the
solvent, a residue of 35.5 g remained. Crystallization from 50 ml of methanol
gave 14.8 g of 6-chloro-α-methylcarbazole-2-acetic acid ethyl ester, MP 106°-
107.5°C (43.2%).
A stirred mixture of 11 g of 6-chloro-α-methylcarbazole-2-acetic acid ethyl
ester, 100 ml ethanol and 100 ml of 3 N sodium hydroxide was heated (N2
atmosphere). After 2 hours at reflux, the reaction mixture was concentrated
to dryness under reduced pressure. Water (300 ml) and ice (200 g) were
added to the residue and concentrated hydrochloric acid was added until the
mixture was strongly acid. The acidic mixture was extracted with ether (3 x
200 ml). The ether extracts were combined, washed by extraction with water
(3 x 100 ml) and dried over anhydrous magnesium sulfate. Following filtration
of the desiccant and evaporation of the solvent, a yield of 9.89 (98.2%) was
obtained. Crystallization from CHCl3 yielded 6.2 g (62.0%) of 6-chloro-α-
methylcarbazole-2-acetic acid, MP 197°-198°C. A second crop of 1.6 g, MP
195°-199°C was obtained from the mother liquors.
Therapeutic Function
Antiinflammatory
Mechanism of action
Carprofeng is a non-narcotic NSAID with analgesic and antipyretic properties. It acts in the same way as most NSAIDs in that Carprofeng acts by inhibiting cyclooxygenase (COX), which selectively inhibits COX-2, thereby inhibiting the release of several prostaglandins involved in the chronic inflammatory response that is thought to be present in canine OA.
Pharmacokinetics
Carprofen is a non-steroidal anti-inflammatory drug (NSAID) of the propionic acid class that includes ibuprofen, naproxen, and ketoprofen. It is no longer used in the clinical setting, but is approved for use in dogs. Carprofen is non-narcotic and has characteristic analgesic and antipyretic activity approximately equipotent to indomethacin in animal models.
Side Effects
Common side effects of Carprofeng in dogs include vomiting, diarrhoea, loss of appetite, constipation, behavioural changes; and in severe cases, black stools, gastrointestinal ulcers and gastrointestinal haemorrhage, jaundice, skin changes, involuntary muscle movements, and kidney damage (increased thirst, increased urination, and refusal to eat).
Veterinary Drugs and Treatments
Carprofen is labeled (in the USA) for the relief of pain and inflammation
in dogs. It may also prove to be of benefit in other species as
well, but data is scant to support its safety beyond very short-term
use at this time. In Europe, carprofen is reportedly registered for
single dose use in cats, but there have been reported problems (e.g.,
vomiting) with cats receiving more than a single dose.
Carprofen is being investigated for antineoplastic effects in dogs
and may be a useful adjunctive treatment for some types of tumors
with COX-2 overexpression.
Metabolism
Hepatic.
Properties of Carprofen
| Melting point: | 186-1880C |
| Boiling point: | 509.1±35.0 °C(Predicted) |
| Density | 1.2011 (rough estimate) |
| refractive index | 1.5200 (estimate) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMSO: soluble20mg/mL, clear |
| form | powder |
| pka | 4.84±0.30(Predicted) |
| color | white to beige |
| Merck | 14,1862 |
| InChI | InChI=1S/C15H12ClNO2/c1-8(15(18)19)9-2-4-11-12-7-10(16)3-5-13(12)17-14(11)6-9/h2-8,17H,1H3,(H,18,19) |
| CAS DataBase Reference | 53716-49-7(CAS DataBase Reference) |
| EPA Substance Registry System | 9H-Carbazole-2-acetic acid, 6-chloro-.alpha.-methyl- (53716-49-7) |
Safety information for Carprofen
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
Computed Descriptors for Carprofen
| InChIKey | PUXBGTOOZJQSKH-UHFFFAOYSA-N |
| SMILES | N1C2=C(C=C(Cl)C=C2)C2=C1C=C(C(C)C(O)=O)C=C2 |
Carprofen manufacturer
Zenfold Sustainable Technologies (ZST)
Adani Wilmar Limited
Alivira Animal Health Ltd
SETV ASRV LLP
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


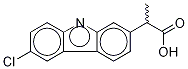
You may like
-
 Carprofen 99%View Details
Carprofen 99%View Details -
 rac-Carprofen 98%View Details
rac-Carprofen 98%View Details
53716-49-7 -
 53716-49-7 Carprofen 98%View Details
53716-49-7 Carprofen 98%View Details
53716-49-7 -
 Carprofen 53716-49-7 99%View Details
Carprofen 53716-49-7 99%View Details
53716-49-7 -
 CARPROFEN 53716-49-7 95-99%View Details
CARPROFEN 53716-49-7 95-99%View Details
53716-49-7 -
 53716-49-7 98%View Details
53716-49-7 98%View Details
53716-49-7 -
 Carprofen 98% CAS 53716-49-7View Details
Carprofen 98% CAS 53716-49-7View Details
53716-49-7 -
 Carprofen CAS 53716-49-7View Details
Carprofen CAS 53716-49-7View Details
53716-49-7