CARBINOXAMINE MALEATE SALT
Synonym(s):Carbinoxamine maleate salt
- CAS NO.:3505-38-2
- Empirical Formula: C20H23ClN2O5
- Molecular Weight: 406.86
- MDL number: MFCD00082461
- EINECS: 222-498-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
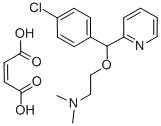
What is CARBINOXAMINE MALEATE SALT?
Description
Carbinoxamine is a competitive histamine H1 receptor antagonist (Ki = 2.3 nM) and first generation antihistamine. It also competes with [3H]diltiazem, an L-type calcium channel blocker, for binding to the benzothiazepine site on rat cardiomyocytes (Ki = 1.08 nM). Carbinoxamine decreases negative inotropic activity in isolated guinea pig left atria by 76% (EC50 = 250 nM) and negative chronotropic activity in guinea pig spontaneously beating isolated right atria (EC50s = 250 and 480 nM, respectively) by 48% compared with control. Formulations containing carbinoxamine have been used in the treatment of allergic rhinitis.
Chemical properties
Off-White Solid
Originator
Clistin,McNeil,US,1953
The Uses of CARBINOXAMINE MALEATE SALT
Analgesic and anti-inflammatory compound
The Uses of CARBINOXAMINE MALEATE SALT
antihistaminic
What are the applications of Application
Carbinoxamine Maleate Salt is an analgesic and anti-inflammatory compound
Definition
ChEBI: The maleic acid salt of carbinoxamine. An ethanolamine-type antihistamine, used for treating hay fever, as well as mild cases of Parkinson's disease.
Manufacturing Process
As described in US Patent 2,800,485 a solution of p-chlorophenylmagnesium
bromide is prepared by adding dropwise a solution of 230 g (1.2 mols) of p-bromochlorobenzene in 900 cc of anhydrous ether to 26.7 g (1.1 g-atoms) of magnesium suspended in 100 cc of anhydrous ether containing a small crystal
of iodine. To this solution, 107 g (1 mol) of 2-pyridinealdehyde are added
slowly with stripping at a rate to maintain refluxing. The reaction mixture is
then stirred for one hour at room temperature. The mixture is then poured
onto an equal volume of crushed ice and water and acidified with concentrated
hydrochloric acid. The ether layer is removed. The aqueous layer is made
basic with ammonia and extracted with ether. The ether solution is evaporated
and the residue dried by addition of benzene and removal by distillation to
give 208 g (95%) of solid alpha-(p-chlorophenyl)-2-pyridinemethanol melting
at 78° to 80°C. The p-chlorophenyl pyridinemethanol may alternatively be
prepared from 4-chloroacetophenone, pyridine and granular aluminum as
described in US Patent 2,606,195. In either case, the synthesis then proceeds
as described in US Patent 2,800,485.
A solution of 219 g (1 mol) of α-(p-chlorophenyl)-2-pyridinemethanol in one
liter of dry toluene is heated to 100°C with stirring. Twenty-three grams (1 g-atom) of sodium are then added in portions. After all the sodium has reacted,
a dried solution of 2-dimethylaminoethyl chloride in benzene is added. This
benzene solution is prepared by dissolving 173 g (1.2 mols) of 2-
dimethylaminoethyl chloride hydrochloride in the minimum amount of water,
adding 500 cc of benzene followed by 300 g of sodium carbonate decahydrate,
stirring, separating the benzene layer and drying.
The mixture is refluxed with stirring for ten hours, cooled and filtered. The
filtrate is extracted three times with 200 cc portions of 6 N acetic acid. The
aqueous acetic acid solution is then made strongly basic with 10% sodium
hydroxide solution, and extracted three times with 200 cc portions of ether.
The ether extract is dried with anhydrous sodium sulfate, stirred with 5 g of
activated carbon and filtered to provide 2-[p-chloro-α(2-dimethylaminoethoxy)
benzyl]pyridine in solution. Addition of a solution of 116 g (1 mol) of maleic
acid in 1,500 cc of ether gives 323 g (79%) of solid which, on recrystallization
from ethyl acetate, gives white solid 2-[p-chloro-α(2-dimethylaminoethoxy)
benzyl]pyridine maleate melting at 117° to 119°C.
brand name
Clistin (Ortho- McNeil).
Therapeutic Function
Antihistaminic
General Description
Carbinoxamine is availableas a bitter bimaleate salt, (d, l)-2-[p-chloro- -[2-(dimethylamino)ethoxy]benzyl]pyridine bimaleate (Clistin), which isa white crystalline powder that is very soluble in water andfreely soluble in alcohol and in chloroform. The pH of a 1%solution is between 4.6 and 5.1.
Carbinoxamine is a potent antihistaminic and is availableas the racemic mixture. It differs structurally from chlorpheniramineonly in having an oxygen atom separate theasymmetric carbon atom from the aminoethyl side chain. Themore active levo isomer of carbinoxamine has the (S) absoluteconfiguration and can be superimposed on the moreactive dextro isomer (S configuration) of chlorpheniramine.
Properties of CARBINOXAMINE MALEATE SALT
| Melting point: | 116-1180C |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| Water Solubility | Completely soluble in water |
| solubility | Chloroform: Slightly Soluble; Methanol: Slightly Soluble |
| form | Solid |
| form | neat |
| color | White to Almost white |
| Merck | 14,1799 |
| CAS DataBase Reference | 3505-38-2(CAS DataBase Reference) |
| EPA Substance Registry System | Ethanamine, 2-[(4-chlorophenyl)-2-pyridinylmethoxy]-N,N-dimethyl-, (2Z)-2-butenedioate (1:1) (3505-38-2) |
Safety information for CARBINOXAMINE MALEATE SALT
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for CARBINOXAMINE MALEATE SALT
| InChIKey | GVNWHCVWDRNXAZ-BTJKTKAUSA-N |
New Products
1-Amino-1-cyclohexanecarboxylic acid Cycloleucine 6-Bromo-3-iodo-1-methyl-1H-indazole 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole ELECTROLYTIC IRON POWDER 2-Methyl-2-phenylpropyl acetate 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride Decanonitrile tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate 4-Ethylbenzylamine Methyl 5-bromo-2-chloro-3-nitrobenzoate N-(5-Amino-2-methylphenyl)acetamide 2-Chloro-3-nitropyridine 5-Bromo-2,3-dimethoxypyridine methyl 6-chloro-2-(chloromethyl)nicotinate 2-methoxy-4-methyl-5-nitro pyridine 2-iodo-5-bromo pyridine 2-amino-4-methyl-5-nitro pyridine 5-Fluoro-2-Oxindole methyl L-alaninate hydrochloride diethyl L-glutamate hydrochloride Ethyl tosylcarbamateRelated products of tetrahydrofuran

You may like
-
 3505-38-2 Carbinoxamine maleate 98%View Details
3505-38-2 Carbinoxamine maleate 98%View Details
3505-38-2 -
 3505-38-2 98%View Details
3505-38-2 98%View Details
3505-38-2 -
 Carbinoxamine maleate 98%View Details
Carbinoxamine maleate 98%View Details
3505-38-2 -
 Carbinoxamine maleate 95% CAS 3505-38-2View Details
Carbinoxamine maleate 95% CAS 3505-38-2View Details
3505-38-2 -
 Carbinoxamine Maleate CAS 3505-38-2View Details
Carbinoxamine Maleate CAS 3505-38-2View Details
3505-38-2 -
 Carbinoxamine maleate CAS 3505-38-2View Details
Carbinoxamine maleate CAS 3505-38-2View Details
3505-38-2 -
 Diethyl Disulfide 99% HPLCView Details
Diethyl Disulfide 99% HPLCView Details
110-81-6 -
 6285-05-8. 1-(4-chlorophenyl) propan-1-one 98%View Details
6285-05-8. 1-(4-chlorophenyl) propan-1-one 98%View Details
6285-05-8.