Candesartan
- CAS NO.:139481-59-7
- Empirical Formula: C24H20N6O3
- Molecular Weight: 440.45
- MDL number: MFCD00864463
- EINECS: 604-138-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
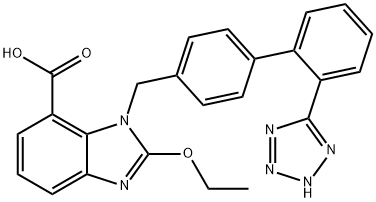
What is Candesartan?
Description
Candesartan (CAS 139481-59-7) is an angiotensin II receptor I (AT1) antagonist, IC50s=1.12 and 2.86 nM for bovine adrenal cortex and rabbit aorta respectively.1?Selectively inhibits angiotensin II-induced contraction of rabbit aortic strips with no effect on contraction induced by other agents such as norepinephrine, KCl, serotonin, PGF2αor endothelin. Prevents astrocyte and microglial activation and neuroinflammation and improves hippocampal neurogenesis.2?Attenuates angiogenesis in hepatocellular carcinoma.3?Clinically useful antihypertensive agent. Ameliorates brain inflammation associated with Alzheimer’s disease.4?Active?in vivo?and orally active.
Chemical properties
Crystalline Solid
The Uses of Candesartan
Candesartan is a selective AT1 (angiotensin II receptor 1) antagonist. Antagonism of angiotensin receptors inhibits vasoconstriction and the production of aldosterone, leading to a decrease in water and sodium concentration in blood plasma. Exhibits antihypertensive effects in animal models. Used in treatment of congestive heart failure, as antihypertensive. Candesartan does not affect cell viability or proliferation but increases the expression of VEGF and interleukin-8 in the cultured medium of KU-19-19 cells. Candesartan (0.1 nM) could reduce the maximal contractile response to angiostensin II by approximately 50%.
The Uses of Candesartan
An angiotensin II type-1 receptor antagonist. Used in treatment of congestive heart failure. Antihypertensive
The Uses of Candesartan
antihypertensive, angiotensin II inhibitor
What are the applications of Application
Candesartan is a selective angiotensin II receptor antagonist
Definition
ChEBI: A benzimidazolecarboxylic acid that is 1H-benzimidazole-7-carboxylic acid substituted by an ethoxy group at position 2 and a ({2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl}methyl) group at position 1. It is a angiotensin eceptor antagonist used for the treatment of hypertension.
brand name
Atacand (AstraZeneca).
General Description
Candesartan, (+)-1-[[(cyclohexyloxy)carbonyl]-oxy]ethyl 2- ethoxy-1-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-1H-benzimidazole-7-carboxylate(Atacand), like losartan, possesses the acidic tetrazole system,which most likely plays a role in binding to the angiotensin IIreceptor similarly to the acidic groups of angiotensin II. Also,the imidazole system has been replaced with a benzimidazolepossessing an ester at position. This ester must be hydrolyzedto the free acid. Fortunately, this conversion takesplace fairly easily because of the carbonate in the ester sidechain. This facilitates hydrolysis of the ester so much thatconversion to the free acid takes place during absorption fromthe gastrointestinal tract.
Storage
Store at RT
References
1) Shibouta?et al.?(1993),?Pharmacological profile of a highly potent and long-acting angiotension II receptor antagonist, 2-ethoxy-1-[[2’-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]-1H-benzimidazol-7-carboxylic acid (CV-11974), and its prodrug, (+/-)-1-(cyclohexyloxycarbonyloxy)-ethyl 2-ethoxy-1-[[2’-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]-1H-benzimidazole-7-carboxylate (TCV-116); J. Pharmacol. Exp. Therap.,?266?114 2) Bhat?et al. (2017),?Angiotensin receptor Blockade by Inhibiting Glial Activation Promotes Hippocampal Neurogenesis Via Activation of Wnt/B-Catenin signaling in hypertension; Mol. Neurobiol.,?55 5282 3) Fan?et al.?(2016),?Candesartan attenuates angiogenesis in hepatocellular carcinoma via downregulating AT1R/VEGF pathway; Biomed. Pharmacother.,?83?704 4) Torika?et al.?(2018),?Candesartan ameliorates brain inflammation associated with Alzheimer’s disease; CNS Neurosci. Ther.?24?231
Properties of Candesartan
| Melting point: | 183-185°C |
| Boiling point: | 754.8±70.0 °C(Predicted) |
| Density | 1.41±0.1 g/cm3(Predicted) |
| RTECS | DD6671000 |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Soluble in DMSO (up to 40 mg/ml) |
| form | solid |
| pka | 2.06±0.10(Predicted) |
| color | White |
| Water Solubility | Soluble in ethyl acetate, methanol, water (<1 mg/ml at 25°C), DMSO (88 mg/ml at 25°C), and ethanol (1 mg/ml at 25°C). |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 2 months. |
| CAS DataBase Reference | 139481-59-7(CAS DataBase Reference) |
Safety information for Candesartan
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H312:Acute toxicity,dermal H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Candesartan
Candesartan manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![Methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate](https://img.chemicalbook.in/CAS/GIF/139481-44-0.gif)
![Ethyl -2-ethoxy-1-[[(2-(1Htetrazol-5-yl)biphenyl-4-yl-) methyl]](https://img.chemicalbook.in/CAS/GIF/139481-58-6.gif)
You may like
-
 139481-59-7 99%View Details
139481-59-7 99%View Details
139481-59-7 -
 Candesartan 98%View Details
Candesartan 98%View Details -
 139481-59-7 Candesartan 98%View Details
139481-59-7 Candesartan 98%View Details
139481-59-7 -
 Candesartan 98%View Details
Candesartan 98%View Details
139481-59-7 -
 Candesartan 139481-59-7 99%View Details
Candesartan 139481-59-7 99%View Details
139481-59-7 -
 Candesartan 95.00% CAS 139481-59-7View Details
Candesartan 95.00% CAS 139481-59-7View Details
139481-59-7 -
 Candesartan >98% (HPLC) CAS 139481-59-7View Details
Candesartan >98% (HPLC) CAS 139481-59-7View Details
139481-59-7 -
 Candesartan Cilexetil Related Compound G CAS 139481-59-7View Details
Candesartan Cilexetil Related Compound G CAS 139481-59-7View Details
139481-59-7
