CALCIUM ARSENATE
- CAS NO.:7778-44-1
- Empirical Formula: AsH3O4.3/2Ca
- Molecular Weight: 398.07
- MDL number: MFCD00049407
- EINECS: 231-904-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-14 15:18:27
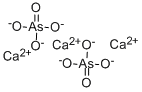
What is CALCIUM ARSENATE?
Description
The calcium arsenate that is sold commercially today as an insecticide is not a single chemical compound but a complex mixture of several calcium arsenates and an excess of calcium hydroxide. The material is made from arsenic trioxide by first oxidizing it to arsenic pentoxide with nitric acid and then reacting the solution of arsenic pentoxide or arsenic acid with a slurry of calcium hydroxide. The conditions of temperature,concentration, and duration of reaction are important because of their influence on the physical nature of the product.
Chemical properties
White powder. Slightly soluble in water; soluble in dilute acids. Decomposes on heating.
Physical properties
Commercial calcium arsenate generally is colored pink and is alkaline in reaction. It is a finely divided powder.
The Uses of CALCIUM ARSENATE
Calcium arsenate has been used extensively against certain insects affecting field crops, especially cotton. It cannot be used safely on apples, peaches, beans, and some other crops because of its burning effect on the foliage and fruit. It is banned by a number of countries.
The Uses of CALCIUM ARSENATE
Insecticide; molluscicide.
The Uses of CALCIUM ARSENATE
Calcium arsenate is also used in weather- resistant wood treatment. It was used mainly in viticulture, in the past. it was mainly used against the boll weevil in cotton cultivation.
Preparation
An old method involving electrolysis in solution for the manufacture of calcium arsenate is as follows. A solution of arsenious oxide in caustic soda (As2O3:NaOH=198:250) is electrolyzed between iron electrodes. Hydrogen and a small quantity of metallic arsenic are liberated at the cathode and very little oxygen at the anode, the basic arsenite in solution being oxidized to arsenate. When this oxidation is complete, any arsenic is filtered off and the solution treated with milk of lime. A basic arsenate of extremely low solubility is precipitated and after removal, is washed and dried.
Definition
ChEBI: Calcium arsenate is a polymer.
General Description
White powder. Slightly soluble in water. Toxic by inhalation and ingestion. Used as an insecticide and germicide.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Has only weak oxidizing power but redox reactions can still occur. Soluble in dilute acids. [Hawley]. Produces toxic fumes of arsenic when heated to decomposition .
Hazard
Toxic by ingestion and inhalation.
Health Hazard
CALCIUM ARSENATE is extremely toxic; the probable oral lethal dose for humans is 5-50 mg/kg, or between 7 drops and 1 teaspoonful for a 150 lb. person. It is an irritant to eyes, respiratory tract, mouth and stomach. Damage to kidneys, liver and the nervous system have been reported. (Non-Specific -- Arsenic) Chronic exposure can cause bone marrow damage, often leading to aplastic anemia. There is epidemiological evidence that chronic ingestion of arsenic compounds causes a predisposition to skin cancers.
Fire Hazard
Fire may produce irritating or poisonous gases. When heated to decomposition, CALCIUM ARSENATE produces toxic fumes of arsenic. Avoid heat. Hazardous polymerization may not occur.
Safety Profile
Confirmed human carcinogen. Poison by ingestion. Moderately toxic by skin contact. When heated to decomposition it emits toxic fumes of arsenic.
Potential Exposure
AgriculturalChemical Tumorigen. Workers engaged in manufacture,formulation, and application of pesticides containing calcium arsenate.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, getmedical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure.Note to physician: For severe poisoning BAL [BritishAnti-Lewisite, dimercaprol, dithiopropanol (C3H8OS2)] hasbeen used to treat toxic symptoms of certain heavy metalspoisoning including arsenic. Although BAL is reported tohave a large margin of safety, caution must be exercised,because toxic effects may be caused by excessive dosage.Most can be prevented by premedication with 1-ephedrinesulfate (CAS: 134-72-5). For milder poisoning penicillamine(not penicillin) has been used, both with mixed success.Side effects occur with such treatment and it is never a substitute for controlling exposure. It can only be done understrict medical care.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with Calciumarsenate you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area. A regulated, marked area should be established where this chemical is handled, used, or stored incompliance with OSHA Standard 1910.1045.
Shipping
The DOT label requirement is “POISONOUS/TOXIC MATERIALS.” The Hazard Class is 6.1 and thePacking Group is II.
Incompatibilities
None reported, according to NIOSH.When heated produces arsenic fumes.
Properties of CALCIUM ARSENATE
| Melting point: | 1455℃ [CRC10] |
| Density | 3,62 g/cm3 |
| vapor pressure | 0Pa at 20℃ |
| RTECS | CG0830000 |
| solubility | soluble in dilute acid solutions |
| form | Powder |
| color | White |
| Specific Gravity | 3.62 |
| Water Solubility | It is soluble in water and acids, insoluble in organic solvents |
| Solubility Product Constant (Ksp) | pKsp: 18.17 |
| Exposure limits | ACGIH: TWA 0.01 mg/m3 NIOSH: IDLH 5 mg/m3; Ceiling 0.002 mg/m3 |
| CAS DataBase Reference | 7778-44-1(CAS DataBase Reference) |
| EPA Substance Registry System | Calcium arsenate (7778-44-1) |
Safety information for CALCIUM ARSENATE
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H331:Acute toxicity,inhalation H350:Carcinogenicity H400:Hazardous to the aquatic environment, acute hazard H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P405:Store locked up. |
Computed Descriptors for CALCIUM ARSENATE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


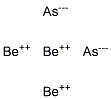


You may like
-
 Calcium arsenate CAS 7778-44-1View Details
Calcium arsenate CAS 7778-44-1View Details
7778-44-1 -
 Calcium arsenate CAS 7778-44-1View Details
Calcium arsenate CAS 7778-44-1View Details
7778-44-1 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4