CALCIFEDIOL
Synonym(s):25-Hydroxycholecalciferol solution;25-Hydroxyvitamin D3
- CAS NO.:19356-17-3
- Empirical Formula: C27H44O2
- Molecular Weight: 400.65
- MDL number: MFCD00867077
- EINECS: 242-990-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-15 19:19:13
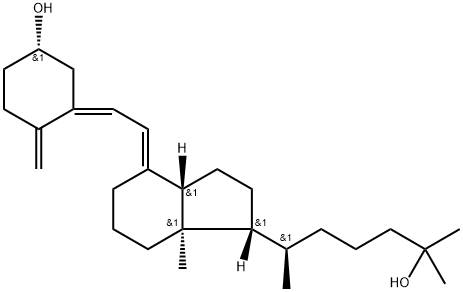
What is CALCIFEDIOL?
Absorption
Readily absorbed.
Toxicity
Bone pain, constipation (especially in children or adolescents), diarrhea, drowsiness, dryness of mouth; headache (continuing), increased thirst, increase in frequency of urination, especially at night, or in amount of urine, irregular heartbeat, itching skin, loss of appetite, metallic taste, muscle pain, nausea or vomiting (especially in children or adolescents), unusual tiredness or weakness.
Chemical properties
White Solid
Originator
Dedrogyl,Roussel,France,1976
The Uses of CALCIFEDIOL
A metabolite of Vitamin D. The principal circulating form of vitamin D3, formed in the liver by hydroxylation at C-25. Calcium regulator.
The Uses of CALCIFEDIOL
25-Hydroxyvitamin D3-23,24,25,26,27-13C5?can be used as a stable isotope internal standard for data interpretation in specific biological samples by mass spectrometry.
The Uses of CALCIFEDIOL
This product may be used as an analytical standard.
Background
The major circulating metabolite of vitamin D3 (cholecalciferol). It is produced in the liver and is the best indicator of the body's vitamin D stores. It is effective in the treatment of rickets and osteomalacia, both in azotemic and non-azotemic patients. Calcifediol also has mineralizing properties.
Indications
Used to treat vitamin D deficiency or insufficiency, refractory rickets (vitamin D resistant rickets), familial hypophosphatemia and hypoparathyroidism, and in the management of hypocalcemia and renal osteodystrophy in patients with chronic renal failure undergoing dialysis. Also used in conjunction with calcium in the management and prevention of primary or corticosteroid-induced osteoporosis.
What are the applications of Application
25-Hydroxyvitamin D3 is a circulating metabolite of vitamin D, and the principal circulating form of vitamin D3.
Definition
ChEBI: A hydroxycalciol that is calciol in which the hydrogen at position 25 has been replaced by a hydroxy group. A prehormone resulting from the oxidation of calciol in the liver, it is further hydroxylated in the kidney to give calcitriol, the active form of v tamin D3.
Manufacturing Process
A solution of 125 mg of cholesta-5,7-diene-3β,25-diol in 125 ml of benzene
and 10 ml of absolute ethanol is placed in a photo reactor equipped with a
quartz lamp well cooled with water and a nitrogen inlet. The reaction mixture
is cooled to about 16°C, and purged with N2. A Hanovia 8A36, 100-watt lamp,
centered in the lampwell 2.5 cm from the internal surface of the reaction
mixture, is turned on for 15 minutes, including the 5-6 minutes required for
the lamp to reach full brilliance. The lamp is a typical actinic energy source
suitable for the irradiation step in the known synthesis of Vitamin D, and can
be replaced by any such available lamp. The specific lamp used is a 100-watt
high-pressure quartz mercury-vapor lamp, producing approximately 11.5
watts total radiated energy distributed over the range of 220-1400 nm. A fast
stream of water is necessary to keep the outlet water temperature below
20°C. The reaction mixture is concentrated to dryness in a rotary evaporator
below room temperature. The semisolid residue is triturated with 5 ml of 35%
ethyl acetate-65% Skellysolve B hexanes mixture and filtered and another 5
ml of the same solvent is used for wash. The solid contains unreacted starting
material and the liquor contains the product. The liquor is poured onto a 40 g
column containing TLC grade Florisil, 150-200 mesh packed wet with 35%
ethyl acetate-Skellysolve B hexanes, and the products are eluted with the
same solvent mixture collecting 10 ml fractions. The fractions containing the
product, located by spotting on a TLC plate, are combined and evaporated to
dryness below room temperature to give an oily residue. A few drops of
absolute ether are added and removed under vacuum to give 25-
hydroxyprecholecalciferol as a fluffy foam; yield 60 mg.
A solution of about 300 mg of 25-hydroxyprecholecalciferol prepared as
described above in 5 ml of chloroform is heated for 3.5 hours at 70°-75°C
under N2 in a sealed flask. The solvent is evaporated and the residue is
chromatographed through a 60 g column containing TLC grade Florisil, 150-
200 mesh packed wet with 35% ethyl acetate in Skellysolve B hexanes. The
column is eluted with the same solvent mixture, collecting 10 ml fractions.
The fractions which crystallize on trituration with aqueous methanol are
combined and recrystallized twice from aqueous methanol to give 25-
hydroxycholecalciferol hydrate; yield 120 mg, MP 81°-83°C (sinters 75°C).
A solution of 20 mg of 25-hydroxycholecalciferol hydrate, prepared as
described above, in 20 ml of methylene chloride is dried with 200 mg of
anhydrous sodium sulfate. The solution is filtered and the filtrate is
evaporated to yield 25-hydroxycholecalciferol essentially anhydrous as an
amorphous oil.
brand name
Calderol (Organon).
Therapeutic Function
Calcium regulator
General Description
25-Hydroxyvitamin D3 (calcifediol) is a prohormone produced via hydroxylation of vitamin D3 (cholecalciferol) in the liver. It is a precursor for the synthesis of calcitriol {1,25-dihydroxyvitamin D3 or [1,25(OH)2D3]}. It is used as a biomarker to determine the status of vitamin D in the body.
25-Hydroxyvitamin D3-23,24,25,26,27-13C5?is an isotope of vitamin D3 wherein C-23, C-24, C-25, C-26, C-27 carbons are replaced by 13C6 isotope.
Biochem/physiol Actions
Ergocalciferol (vitamin D2) and 25-Hydroxycholecalciferol (vitamin D3) are the two form of vitamin D which are activated in vivo by hydroxylation. Vitamin D2 and D3 may be used in a wide range of studies to assess their effects on function such as immune function and calcium homeostasis.
Pharmacokinetics
Calcidiol is the precursor of vitamin D3. Vitamin D3 is a steroid hormone that has long been known for its important role in regulating body levels of calcium and phosphorus, in mineralization of bone, and for the assimilation of vitamin A. The classical manifestations of vitamin D deficiency is rickets, which is seen in children and results in bony deformaties including bowed long bones. Deficiency in adults leads to the disease osteomalacia. Both rickets and osteomalacia reflect impaired mineralization of newly synthesized bone matrix, and usually result from a combination of inadequate exposure to sunlight and decreased dietary intake of vitamin D. Common causes of vitamin D deficiency include genetic defects in the vitamin D receptor, severe liver or kidney disease, and insufficient exposure to sunlight. Vitamin D plays an important role in maintaining calcium balance and in the regulation of parathyroid hormone (PTH). It promotes renal reabsorption of calcium, increases intestinal absorption of calcium and phosphorus, and increases calcium and phosphorus mobilization from bone to plasma.
Metabolism
Calcidiol undergoes hydroxylation in the mitochondria of kidney tissue, and this reaction is activated by the renal 25-hydroxyvitamin D3-1-(alpha)-hydroxylase to produce calcitriol (1,25- dihydroxycholecalciferol), the active form of vitamin D3.
Properties of CALCIFEDIOL
| Melting point: | 74-76oC |
| Boiling point: | 529.2±33.0 °C(Predicted) |
| Density | 1.01±0.1 g/cm3(Predicted) |
| Flash point: | 14 °C |
| storage temp. | -20°C |
| solubility | DMF: 20 mg/ml; DMSO: 10 mg/ml; Ethanol: 20 mg/ml; Ethanol:PBS (pH 7.2)(1:2): 0.3 mg/ml |
| pka | 14.74±0.20(Predicted) |
| form | White to off-white crystalline solid. |
| color | White to off-white |
| Stability: | Light Sensitive, Temperature Sensitive |
Safety information for CALCIFEDIOL
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for CALCIFEDIOL
| InChIKey | JWUBBDSIWDLEOM-DTOXIADCSA-N |
CALCIFEDIOL manufacturer
Dishman Carbogen Amcis Ltd (Dishman Group)
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
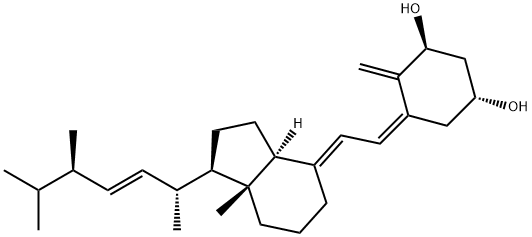
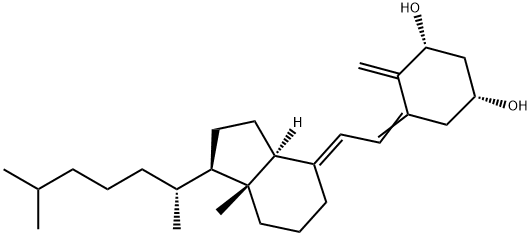
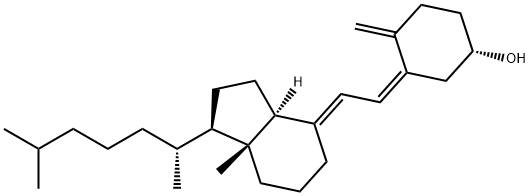
You may like
-
 19356-17-3 Calcifediol 98%View Details
19356-17-3 Calcifediol 98%View Details
19356-17-3 -
 Calcifediol 95% CAS 19356-17-3View Details
Calcifediol 95% CAS 19356-17-3View Details
19356-17-3 -
 25-Hydroxyvitamin D3 solution CAS 19356-17-3View Details
25-Hydroxyvitamin D3 solution CAS 19356-17-3View Details
19356-17-3 -
 25-Hydroxyvitamin D3 solution CAS 19356-17-3View Details
25-Hydroxyvitamin D3 solution CAS 19356-17-3View Details
19356-17-3 -
 25-Hydroxycholecalciferol CAS 19356-17-3View Details
25-Hydroxycholecalciferol CAS 19356-17-3View Details
19356-17-3 -
 25-Hydroxyvitamin D3-23,24,25,26,27-13C5 solution CAS 19356-17-3View Details
25-Hydroxyvitamin D3-23,24,25,26,27-13C5 solution CAS 19356-17-3View Details
19356-17-3 -
 25-Hydroxyvitamin D3 solution CAS 19356-17-3View Details
25-Hydroxyvitamin D3 solution CAS 19356-17-3View Details
19356-17-3 -
 Vitamin D3, 25-Hydroxy- CAS 19356-17-3View Details
Vitamin D3, 25-Hydroxy- CAS 19356-17-3View Details
19356-17-3