Cadmium fluoride
- CAS NO.:7790-79-6
- Empirical Formula: CdF2
- Molecular Weight: 150.41
- MDL number: MFCD00010919
- EINECS: 232-222-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-28 13:53:28
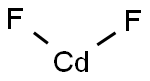
What is Cadmium fluoride ?
Chemical properties
White powder
Physical properties
Colorless cubic crystal; density 6.33 g/cm3; melts at 1,110°C; vaporizes at 1,748°C; vapor pressure 5 torr at 1,231°C; moderately soluble in water, 4.35 g/100mL at 25°C; soluble in hydrofluoric and other mineral acids; practically insoluble in alcohol and liquid ammonia.
The Uses of Cadmium fluoride
Cadmium fluoride is used in phosphors, glass and nuclear reactor controls. It is also used in high-temperature lubricants and to start crystals for lasers. It is used in oxygen-sensitive applications such as the production of metallic alloys and it is also used in limited medical treatment protocols. In addition, it can be used in synthetic organic chemistry.
The Uses of Cadmium fluoride
Manufacture of phosphors, glass; in nuclear reactor controls.
Definition
Available as pure crystals, 99.89%, d 6.6, mp approx- imately 1110C, soluble in water and acids, insoluble in alkalies.
Preparation
Cadmium fluoride is prepared by the reaction of gaseous fluorine or hydrogen fluoride with cadmium metal or its salt, such as chloride, oxide or sulfide:
Cd + F2 → CdF2
Cd + 2HF → CdF2 + H2
CdO + 2HF → CdF2 + H2O
It also may be obtained by dissolving cadmium carbonate in 40% hydrofluoric acid solution, evaporating the solution and drying in vacuum at 150°C:
CdCO3 + 2HF → CdF2 + H2O + CO2
It also may be prepared by mixing cadmium chloride and ammonium fluoride solutions, followed by crystallization.
Safety Profile
Confirmed human carcinogen. Poison by subcutaneous route. Violent reaction with K. When heated to decomposition it emits very toxic fumes of Cd and F-. See also FLUORIDES and CADMIUM COMPOUNDS.
Purification Methods
Crystallise it by dissolving it in hot water (25mL/g at room temperature) at 60o, filtering, then cooling. [Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 243 1963.]
Properties of Cadmium fluoride
| Melting point: | 1049 °C |
| Boiling point: | 1748 °C |
| Density | 6.33 g/mL at 25 °C(lit.) |
| Flash point: | 1758°C |
| solubility | soluble in acid solutions; insoluble in ethanol |
| form | Powder |
| color | white |
| Specific Gravity | 6.64 |
| Water Solubility | 4.3 g/100 mL (25 ºC) |
| Merck | 14,1619 |
| Solubility Product Constant (Ksp) | pKsp: 2.19 |
| CAS DataBase Reference | 7790-79-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Cadmium difluoride(7790-79-6) |
| EPA Substance Registry System | Cadmium fluoride (CdF2) (7790-79-6) |
Safety information for Cadmium fluoride
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H330:Acute toxicity,inhalation H340:Germ cell mutagenicity H350:Carcinogenicity H360:Reproductive toxicity H372:Specific target organ toxicity, repeated exposure H400:Hazardous to the aquatic environment, acute hazard H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P320:Specific treatment is urgent (see … on this label). P330:Rinse mouth. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P405:Store locked up. |
Computed Descriptors for Cadmium fluoride
New Products
4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION Methylcobalamin (vitamin B12) SODIUM METHYL PARABEN SODIUM VALPROATE AMOXICILLIN (AMOXYCILLIN) TRIHYDRATE ACICLOVIRRelated products of tetrahydrofuran

You may like
-
 Cadmium fluoride CAS 7790-79-6View Details
Cadmium fluoride CAS 7790-79-6View Details
7790-79-6 -
 Cadmium fluoride CAS 7790-79-6View Details
Cadmium fluoride CAS 7790-79-6View Details
7790-79-6 -
 Cadmium fluoride CAS 7790-79-6View Details
Cadmium fluoride CAS 7790-79-6View Details
7790-79-6 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9