Potassium fluoride
Synonym(s):Potassium Fluoride;Potassium monofluoride;Potassium monofluoride (KF)
- CAS NO.:7789-23-3
- Empirical Formula: FK
- Molecular Weight: 58.1
- MDL number: MFCD00011398
- EINECS: 232-151-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:44
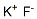
What is Potassium fluoride?
Chemical properties
White, crystalline, deliquescent powder; sharp saline taste.Soluble in water and hydrogen fluoride, insoluble in alcohol.
Chemical properties
Potassium fluoride is a white crystalline solid.
The Uses of Potassium fluoride
The Uses of Potassium fluoride
Potassium fluoride is used in metal finishing, batteries, coatings and photographic chemicals. It is utilized for the study of ion-specific swelling and de-swelling of ampholytic polymer gels as well as in the measurement of electronic polarizabilities of ions in polymers of alkali halides. It finds application in the electronic industry as a metal surface treatment product. It is used as a preservative, a food additive, a catalyzer and a water absorbing agent. In the Finkelstein reaction, it is actively involved in the conversion of chlorocarbons to fluorocarbons using polar solvents like dimethyl formamide and ethylene glycol.
Definition
ChEBI: Potassium fluoride is a fluoride salt having K+ as the counterion. It has a role as a poison. It is a fluoride salt and a potassium salt.
Reactivity Profile
Potassium fluoride reacts with acids to evolve corrosive and toxic hydrogen fluoride. Aqueous solutions corrode glass and consequently are prepared and stored in polyethylene containers. The pure solid may be stored in glass containers. Reacts violently with (Pt + BrF3). [NTP 1992].
Hazard
Toxic by ingestion and inhalation, strong irritant to tissue.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
Poison by ingestion and intraperitoneal routes. Moderately toxic by subcutaneous route. Experimental teratogenic effects. A corrosive irritant to the eyes, skin, and mucous membranes. Mutation data reported. A very reactive material. When heated to decomposition it emits toxic fumes of K2O and F-. Used in etchmg glass, as a presewative, as an insecticide, and in organic synthesis. See also FLUORIDES and HYDROFLUORIC ACID.
Potential Exposure
Potassium fluoride is used in etching glass; as a preservative and insecticide.
Shipping
UN1812 Potassium fluoride, solid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Incompatible with strong acids; reacts releasing hydrogen fluoride. Aqueous solutions corrode glass and consequently are prepared and stored in polyeth- ylene containers. The pure solid may be stored in glass containers. Reacts violently with (Pt 1 BrF 3 )
Properties of Potassium fluoride
| Melting point: | 858 °C (lit.) |
| Boiling point: | 1505 °C |
| Density | 2.48 |
| vapor density | 2 (vs air) |
| vapor pressure | 1.3 hPa (885 °C) |
| refractive index | 1.363 |
| Flash point: | 1505°C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | spray-dried |
| appearance | White powder |
| Specific Gravity | 2.481 |
| color | White |
| Odor | Odorless |
| PH | 7.0-8.5 (25℃, 1M in H2O) |
| Water Solubility | 92.3 g/100 mL (18 ºC) |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.01 λ: 280 nm Amax: 0.01 |
| Merck | 14,7632 |
| BRN | 3902818 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| Stability: | Stable. Protect from moisture. Incompatible with strong acids, strong bases. |
| CAS DataBase Reference | 7789-23-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Potassium fluoride(7789-23-3) |
| EPA Substance Registry System | Potassium fluoride (7789-23-3) |
Safety information for Potassium fluoride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Potassium fluoride
| InChIKey | NROKBHXJSPEDAR-UHFFFAOYSA-M |
Potassium fluoride manufacturer
JSK Chemicals
Zama Chemical
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Potassium Fluoride 99%View Details
Potassium Fluoride 99%View Details -
 Potassium Fluoride 99%View Details
Potassium Fluoride 99%View Details -
 Potassium fluoride 98%View Details
Potassium fluoride 98%View Details -
 Potassium fluoride 98%View Details
Potassium fluoride 98%View Details -
 Potassium fluoride 98%View Details
Potassium fluoride 98%View Details -
 Potassium fluoride, anhydrous CAS 7789-23-3View Details
Potassium fluoride, anhydrous CAS 7789-23-3View Details
7789-23-3 -
 Potassium fluoride, 98% 99%View Details
Potassium fluoride, 98% 99%View Details -
 potassium fluoride CASView Details
potassium fluoride CASView Details
