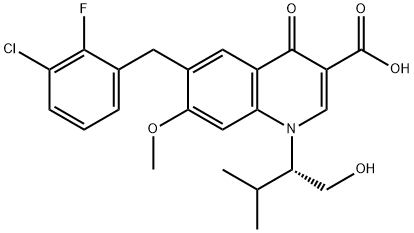
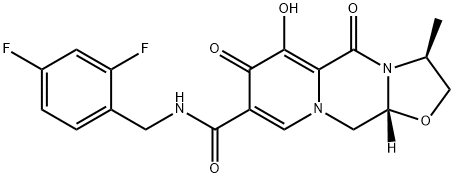
Cabotegravir (GSK744, GSK1265744)
- CAS NO.:1051375-10-0
- Empirical Formula: C19H17F2N3O5
- Molecular Weight: 405.35
- MDL number: MFCD25976748
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
What is Cabotegravir (GSK744, GSK1265744)?
Absorption
Oral cabotegravir has a Tmax of 3 hours, reaches a Cmax of 8.0 μg/mL, and has an AUC of 145 μg*h/mL. Intramuscular extended-release cabotegravir has a Tmax of 7 days, reaches a Cmax of 8.0 μg/mL, and has an AUC of 1591 μg*h/mL.
Toxicity
Data regarding the toxicity of cabotegravir is not readily available. In the event of overdose, patients should have their vital signs monitored, including an ECG to monitor the QT interval. Treat patients symptomatically and supportively. As cabotegravir is highly protein bound, dialysis is not expected to remove a significant amount of the drug from plasma.
The Uses of Cabotegravir (GSK744, GSK1265744)
Cabotegravir is a long acting HIV-1 integrase inhibitor with action against a broad range of HIV subtypes.
Indications
Oral cabotegravir is indicated in combination with rilpivirine for the short-term treatment of HIV-1 in virologically suppressed adults with no history of treatment failure to assess tolerability of cabotegravir or who have missed an injected dose of cabotegravir. Intramuscular extended-release cabotegravir in combination with rilpivirine is indicated as a complete regimen for the treatment of HIV-1 infection in adults and adolescents 12 years of age and older weighing at least 35 kg to replace the current antiretroviral regimen in those who are virologically suppressed (HIV-1 RNA <50 copies/mL) on a stable antiretroviral regimen with no history of treatment failure and with no known or suspected resistance to either cabotegravir or rilpivirine.
An extended-release injectable suspension formulation of cabotegravir is also indicated for the prevention of sexually-acquired HIV-1 infection (i.e. for pre-exposure prophylaxis, PrEP) in at-risk adults and adolescents weighing at least 35kg.
Background
Cabotegravir, or GSK1265744, is an HIV-1 integrase inhibitor that is prescribed with the non-nucleoside reverse transcriptase inhibitor, rilpivirine. Early research into cabotegravir showed it had lower oral bioavailability than dolutegravir, which resulted in the development of long acting monthly intramuscular injection formulation for cabotegravir.
Cabotegravir was granted FDA approval on 21 January 2021 in combination with rilpivirine to treat HIV-1 infection in virologically suppressed individuals. While previously administered once monthly only, this combination product was granted FDA approval for dosing every two months on February 01, 2022 and without the need for an oral lead-in period prior.
Definition
ChEBI: Cabotegravir is a monocarboxylic acid amide obtained by formal condensation of the carboxy group of (3S,11aR)-6-hydroxy-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydro[1,3]oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxylic acid with the amino group of 2,4-difluorobenzylamine. Used (as its sodium salt) for treatment of HIV-1. It has a role as a HIV-1 integrase inhibitor. It is a difluorobenzene, a secondary carboxamide, a monocarboxylic acid amide and an organic heterotricyclic compound. It is a conjugate acid of a cabotegravir(1-).
Biological Activity
Cabotegravir (GSK744, GSK1265744) is a long-acting HIV integrase inhibitor against a broad range of HIV subtypes, inhibiting HIV-1 integrase-catalyzed strand transfer with IC50 of 3 nM. Phase 2.
Mechanism of action
Caboggravir is a potent inhibitor of HIV integrase, which prevents the HIV virus from infecting human cells, while rilpivirine prevents the virus from replicating itself.
Pharmacokinetics
Cabotegravir is an inhibitor of HIV integrase, which reduces viral replication. It has a long duration of action as the oral tablet is given daily and the intramuscular suspension is given monthly. Patients should be counselled regarding the risk of hypersensitivity, hepatotoxicity, and depression.
Metabolism
Cabotegravir is O-glucuronidated to the M1 and M2 metabolites, with 67% of glucuronidation performed by UGT1A1, and 33% by UGT1A9.
Properties of Cabotegravir (GSK744, GSK1265744)
| Boiling point: | 664.0±55.0 °C(Predicted) |
| Density | 1.57±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | insoluble in H2O; insoluble in EtOH; ≥19.35 mg/mL in DMSO with gentle warming |
| form | solid |
| pka | 4.50±1.00(Predicted) |
| color | White to yellow |
Safety information for Cabotegravir (GSK744, GSK1265744)
Computed Descriptors for Cabotegravir (GSK744, GSK1265744)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 1051375-10-0 Cabotegravir 98%View Details
1051375-10-0 Cabotegravir 98%View Details
1051375-10-0 -
 1051375-10-0 Cabotegravir 98%View Details
1051375-10-0 Cabotegravir 98%View Details
1051375-10-0 -
 Cabotegravir 95% CAS 1051375-10-0View Details
Cabotegravir 95% CAS 1051375-10-0View Details
1051375-10-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8