Butyl 4,4-bis(tert-butyldioxy)valerate
- CAS NO.:995-33-5
- Empirical Formula: C17H34O6
- Molecular Weight: 334.45
- MDL number: MFCD00072669
- EINECS: 213-626-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
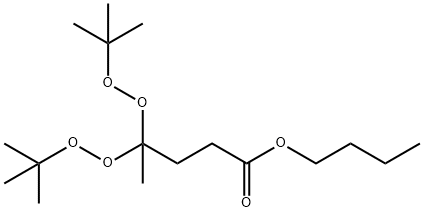
What is Butyl 4,4-bis(tert-butyldioxy)valerate?
Chemical properties
n-butyl-4,4-bis(tert-butylperoxy) valerate is a colorless to straw liquid, with slight bleach-like odor. The product is a colorless liquid with slight camphor odor, chemically unstable (should only be handled under specified conditions, below 38°C/100°F). Contact with incompatible materials or exposure to temperatures exceeding the self accelerating decomposition temperature may result in a self-accelerating decomposition reaction with release of flammable vapors that may autoignite.
The Uses of Butyl 4,4-bis(tert-butyldioxy)valerate
Butyl 4,4'-bis(tert-butylperoxy)valerate acts as a crosslinker peroxide agent in the synthesis of polymers.
General Description
Butyl 4,4-bis(tert-butyldioxy)valerate is sensitive to heat. Storage of Butyl 4,4-bis(tert-butyldioxy)valerate must be done so with stringent temperature control measures. It's explosion hazard is also mitigated by mixing the peroxide with an inert solid.
Reactivity Profile
Peroxides, such as N-BUTYL-4,4-DI-(TERT-BUTYLPEROXY)VALERATE, are good oxidizing agents. Organic compounds can ignite on contact with concentrated peroxides. Strongly reduced material such as sulfides, nitrides, and hydrides may react explosively with peroxides. There are few chemical classes that do not at least produce heat when mixed with peroxides. Many produce explosions or generate gases (toxic and nontoxic). Generally, dilute solutions of peroxides (<70%) are safe, but the presence of a catalyst (often a transition metal such as cobalt, iron, manganese, nickel, or vanadium) as an impurity may even then cause rapid decomposition, a buildup of heat, and even an explosion. Solutions of peroxides often become explosive when evaporated to dryness or near-dryness. Danger of explosion when dry. May explode from heat, shock, friction or contamination. May ignite combustibles (wood, paper, oil, clothing, etc.). May be ignited by heat, sparks or flames.
Properties of Butyl 4,4-bis(tert-butyldioxy)valerate
| Boiling point: | 363.9±37.0 °C(Predicted) |
| Density | 0.9669 g/cm3 |
| vapor pressure | 0.053Pa at 25℃ |
| solubility | Chloroform (Slightly), DMSO (Slightly), Ethyl Acetate (Slightly) |
| form | Oil |
| color | Colourless |
| Water Solubility | 2.16mg/L at 20.2℃ |
| Stability: | Possibly Shock Sensitive |
| EPA Substance Registry System | Butyl 4,4-di(tert-butylperoxy)valerate (995-33-5) |
Safety information for Butyl 4,4-bis(tert-butyldioxy)valerate
Computed Descriptors for Butyl 4,4-bis(tert-butyldioxy)valerate
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1