Butofilolol
- CAS NO.:64552-17-6
- Empirical Formula: C17H26FNO3
- Molecular Weight: 311.394
- MDL number: MFCD00864575
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:41
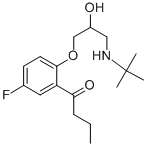
What is Butofilolol?
Originator
Cafide,Clin Midy,France,1982
The Uses of Butofilolol
Butofilolol is a beta-blocker drug and used for treating hypertension.
Definition
ChEBI: Butofilolol is an aromatic ketone.
Manufacturing Process
(a) 5-Chloromethyl-3-tert-butyl-2-(2-hydroxy-5-fluorophenyl)oxazolidine: 5-
Fluorosalicylaldehyde (1.4 g, 0.01 mol) is dissolved in anhydrous benzene (20
ml) in the presence of a crystal of p-toluenesulfonic acid in a Dean-Stark
apparatus. 1-Chloro-2-hydroxy-3-tert-butylaminopropane (2.08 g,
approximately 1 equivalent, purity 75%) is then added within a period of 10
hours in portions of 250 mg at a time at the reflux temperature of benzene
and the mixture is allowed to stand overnight. An insoluble substance is
precipitated on addition of ether after which the solution is filtered,
concentrated and distilled. A fraction is obtained having a boiling point of
118°-123°C/10-3 mm of mercury. A mixture of 1.03 g (yield 43%) of isomeric
oxazolidines is obtained which solidifies. This is crystallized once from hexane.
Melting point 75°-78°C.
(b) 8-Aza-4,9-dioxa-11l-fluoro-8-tert-butyl-2,3-benzobicyclo[4.2.1]octane:
The product of the previous stage (620 mg) is dissolved in anhydrous
dimethylformamide (10 ml) and two quantities each of 300 mg of 50%
sodium hydride is added within 2 hours. The mixture is then left for 24 hours
at 25°C while being stirred mechanically and is then heated for 2 minutes on
a water bath (80°-90°C). The mixture is poured into water, the product
extracted with ether, the ethereal extract dried over anhydrous sodium sulfate
and the organic phase then concentrated and filtered through a short column
of activated alumina. A mixture of light petroleum and diethyl ether (75:25) is
used to elute 186 mg of pure product from the column. Melting point 85°-
86°C (after recrystallization from diisopropyl ether).
(c) 1-(2-Formyl-4-fluorophenoxy)-2-hydroxy-3-tert-butylaminopropane: The
compound obtained as described above (50 mg) is dissolved in a solution of
1N hydrochloric acid (0.5 ml). The mixture is then heated on a water bath
(80°-90°C) for several hours. After complete hydrolysis, which requires
approximately 8 hours, the mixture is poured into an excess of water which
has been basified, the solid base thus formed is extracted with ether, dried
and recrystallized from diisopropyl ether. Melting point 103°-105°C.
(d)1-[2(1-Hydroxybutyl)-4-fluorophenoxy]-2-hydroxy-3-tert-butylaminopropane: To a solution of propylmagnesium bromide prepared from
195 mg (8.1 X 10-3 mol) of magnesium, 1.08 g (8.1 X 10-3 mol) of
bromopropane and a crystal of iodine in 10 ml of anhydrous diethyl ether
under nitrogen is added a solution of the previously prepared aldehyde (197
mg, 0.73 x 10-3 mol) in 4 ml of an ether/tetrahydrofuran mixture (1:3 by
volume) and the mixture is heated to reflux for 70 minutes. The mixture is
poured into water, extracted with diethyl ether, dried over anhydrous sodium
sulfate and 208 mg of an oil which is homogeneous, as shown by thin-layer
chromatography, is isolated.
(e) CM 6805 (Butofilolol): The previously prepared base (200 mg, 0.66 X mol)
is dissolved in purified acetone (8 ml). A drop of sulfuric acid solution
(prepared from 35 ml of concentrated sulfuric acid and 65 ml of water) is
added and the mixture heated on a water bath for 1 minute. When the
solution has cooled to 5° to 10°C a solution of chromic acid (66 mg, 1
equivalent) dissolved in 2 ml of the same acid solution is quickly added and
the resulting mixture is stirred while cold. The mixture is then poured into a
saturated solution of sodium carbonate, the acetone is evaporated under reduced pressure on a water bath, and the organic phase is extracted with
diethyl ether. After drying and evaporating the solvent an oil is obtained (172
mg) all of which solidifies. Recrystallization is carried out from diisopropyl
ether. 122 mg of CM 6805 is obtained (yield 61%). Melting point 88°-89°C.
Therapeutic Function
Beta-adrenergic blocker
Properties of Butofilolol
| Melting point: | 88-89° |
Safety information for Butofilolol
Computed Descriptors for Butofilolol
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![[3-(4-FLUOROPHENOXY)PROPYL]METHYLAMINE](https://img.chemicalbook.in/CAS/GIF/883542-69-6.gif)

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1