Bufexamac
Synonym(s):2-(p-Butoxyphenyl)acetohydroxamic acid
- CAS NO.:2438-72-4
- Empirical Formula: C12H17NO3
- Molecular Weight: 223.27
- MDL number: MFCD00078936
- EINECS: 219-451-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:17
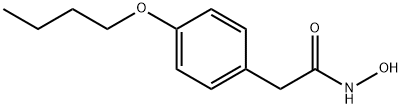
What is Bufexamac?
Absorption
Method of application affects the extent of cutaneous absorption . Following rectal administration as suppositories, the systemic absorption was reported to be low .
Toxicity
The LD50 in rat following oral and intraperitoneal administration is 3370 mg/kg and 805 mg/kg, respectively . Subcutaneous LD50 in mouse is >5000 mg/kg . Mild skin irritation was seen in rabbits following dermal application of 750 mg/30d(l) . Non-steroidal anti-inflammatory drug (NSAID) overdose may produce nausea, vomiting, indigestion and upper abdominal pain. Other effects may include drowsiness, dizziness, confusion, disorientation, lethargy .
Chemical properties
White Solid
Originator
Parfenac,Lederle,UK,1973
The Uses of Bufexamac
Bufexamac is a non-steroidal topical medication used for treatment of a variety of problems in which the skin is inflamed. These vary widely from insect bites to bums, plant stings and a wide variety of medical conditions induding psoriasis, hemorrhoidal symptoms, eczema and inflammation of the skin following radiotherapy. In this last case, it can be used before radiotherapy to help prevent inflammation of the skin caused by radiation therapy.
The Uses of Bufexamac
antiinflammatory, analgesic, antipyretic
The Uses of Bufexamac
Bufexamac is a specific inhibitor of class IIB histone deacetylases (HDAC6 and HDAC10). Bufexamac is a non-steroidal anti-inflammatory drug used topically as well as rectally. Formulations containing Bufexamac is used by many patients with eczematous disorders as an alternative to topical corticosteroids.
What are the applications of Application
Bufexamac is a HDAC6 and HDAC10 inhibitor
Background
Bufexamac is a non-steroidal anti-inflammatory drug (NSAID) under the market name Droxaryl, Malipuran, Paraderm and Parfenac. It is typically administered topically for the treatment of subacute and chronic eczema of the skin, including atopic eczema and other inflammatory dermatoses, as well as sunburn and other minor burns, and itching. It has also been used in suppositories in combination with local anaesthetics indicated for haemorrhoids. The use of bufexamac has been discontinued in Canada and the United States, which may be due to undetermined clinical efficacy and a high prevalence of contact sensitization . Bufexamac was also withdrawn by the EMA in April 2010.
Indications
Indicated for the treatment of various skin conditions, such as atopic eczema and other inflammatory dermatoses.
Definition
ChEBI: A hydroxamic acid derived from phenylacetamide in which the benzene moiety is substituted at C-4 by a butoxy group. It has anti-inflammatory, analgesic, and antipyretic properties.
Manufacturing Process
(1) 136 g of p-hydroxyacetophenone, 140 g of butyl bromide, 152 g of
potassium carbonate, 17 g of potassium iodide and 275 cc of ethanol are
mixed and then refluxed for 48 hours. The reaction mixture is cooled, diluted
with water, then extracted with ether. The ethereal phase is washed with a 10% sodium hydroxide solution, then with water, followed by drying, ether is
evaporated and the product distilled under reduced pressure. 168 g of pbutyloxyacetophenone
are obtained with yield of 87% (160°-162°C at 11 mm
Hg).
(2) 192 g of p-butyloxyacetophenone, 42 g of sulfur and 130 g of morpholine
are mixed and then refluxed for 14 hours. The resulting solution is poured into
water and stirred until crystallization of the sulfurated complex. The latter is
filtered, washed with water and dried, Production: 270 g (88% yield).
(3) 200 g of sodium hydroxide are dissolved in 1,500 cc of ethanol and then
293 g of the thus-obtained sulfurated complex are added. The mixture is
refluxed overnight, The mixture is distilled to separate the maximum of the
alcohol and then diluted with water. The resulting solution is acidified with
hydrochloric acid, and extracted with ether. The ethereal phase is washed with
water, followed by extraction with a 10% sodium carbonate solution. The
carbonated solution is acidified with 10% hydrochloric acid, and the resulting
precipitate of p-n-butyloxyphenylacetic acid is filtered and dried. 100 g of this
product are obtained (70% yield).
(4) 208 g of p-n-butyloxyphenylacetic acid, 368 g of ethanol and 18 cc of
sulfuric acid are refluxed for 5 hours. The mixture is diluted with water, after
which it is extracted with ether. The ethereal phase is successively washed
with water, then with carbonate, and again with water, following which it is
dried and distilled to remove solvent. The ester is then distilled at a reduced
pressure. 200 g of ethyl p-butyloxyphenylecetate are thus obtained with yield
of 61% (186°C at 8 mm Hg).
(5) 7 g of hydroxylamine hydrochloride are dissolved in 100 cc of methanol. A
solution of 5 g of sodium in 150 cc of methanol is added and the salt
precipitate is separated by filtration. 22 g of ethyl p-n-butyloxyphenylacetate
are added to the filtrate and the mixture is refluxed for 1 hour. The mixture is
cooled and acidified with 20% hydrochloric acid. 14.7 g of p-nbutyloxyphenylacetohydroxamic
acid are thus obtained with yield of 71%
(melting point: 153°-155°C).
brand name
Anderm (Wyeth-Ayerst); Paraderm (Wyeth-Ayerst); Parfenac (Wyeth-Ayerst);Bufemac;Bufexamac-ratiopharm (r) creme;Bufexine ratiopharm(r) f-sable;Calmaderm;Droxan;Droxaryl zalf 50 mg;Duradermal;Flogocid gel n.n;Flogocid sable;Malipuran;Parafenac (r) milch;Parafenac 5% creme;Parafenac basishad;Parafenac sable;Parafenal;Parfenal creme derm;Viafen u est.crema 40 g.
Therapeutic Function
Antiinflammatory, Analgesic, Antipyretic
World Health Organization (WHO)
Bufexamac, an analgesic and anti-inflammatory agent, was introduced in 1974 for the topical treatment of a wide range of dermatoses. The drug is widely marketed and the World Health Organization is not aware of restrictive action having been taken elsewhere.
Contact allergens
Bufexamac is an arylacetic nonsteroidal anti-inflammatory drug. It induces allergic contact dermatitis, eczematous or erythema multiforme like type, and even generalized eruptions like acute generalized exanthematous pustulosis.
Pharmacokinetics
Bufexamac is a topically-active anti-inflammatory agent that inhibits the cyclooxygenase enzyme. In cutaneous and deep experimental inflammation, topical administration of bufexamac exerted a dose-related anti-inflammatory effect . In guinea pigs, bufexamax was shown to be more active than topical acetylsalicylic acid 5% or phenylbutazone 5% in delaying the local increase in temperature resulting from UV exposure . Bufexamac is unlikely to have any effect on wound healing .
Metabolism
No data available.
Properties of Bufexamac
| Melting point: | 153-155° |
| Boiling point: | 364.56°C (rough estimate) |
| Density | 1.1223 (rough estimate) |
| refractive index | 1.5300 (estimate) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Practically insoluble in water, soluble in dimethylformamide, slightly soluble in ethyl acetate and in methanol. |
| form | neat |
| pka | 9.24±0.20(Predicted) |
| form | Solid |
| color | White to Off-White |
| Merck | 14,1474 |
| CAS DataBase Reference | 2438-72-4(CAS DataBase Reference) |
Safety information for Bufexamac
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P501:Dispose of contents/container to..… |
Computed Descriptors for Bufexamac
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Bufexamac CAS 2438-72-4View Details
Bufexamac CAS 2438-72-4View Details
2438-72-4 -
 Bufexamac >98% (HPLC) CAS 2438-72-4View Details
Bufexamac >98% (HPLC) CAS 2438-72-4View Details
2438-72-4 -
 Bufexamac CAS 2438-72-4View Details
Bufexamac CAS 2438-72-4View Details
2438-72-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1