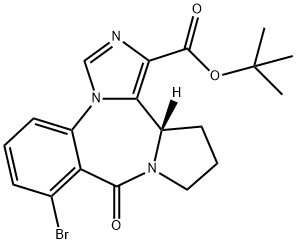
Bretazenil
- CAS NO.:84379-13-5
- Empirical Formula: C19H20BrN3O3
- Molecular Weight: 418.28
- MDL number: MFCD00866987
- EINECS: 240-723-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-04-12 23:00:59
What is Bretazenil?
Description
Bretazenil is an imidazobenzodiazepine partial agonist and a potent anxiolytic. It is rapidly absorbed and has a half-life of 2.5 hours. Animal work has shown that, even after long-term administration of bretazenil, there are no changes in GABA receptor binding or function, and tolerance does not occur. Studies in humans have also suggested that bretazenil has a low abuse potential (Sellers et al. 1991). In terms of clinical effects, bretazenil has demonstrated anxiolytic efficacy in both generalized anxiety disorder and panic disorder (Katschnig et al. 1991), but no controlled study has compared bretazenil with a classic full agonist.
Originator
Bretazenil,Hoffman-La Roche, Inc.
The Uses of Bretazenil
Anti-anxiety agent.
Bretazenil potentiated GABA responses on α4β3γ2 cells.
Bretazenil is a partial benzodiazepine receptor agonist. Benzodiazepines, mainly diazepam, are commonly used as anticonvulsants in the treatment of organophosphate casualties. Bretazenil has been used to determine non-specific binding due to its affinity to bind to a variety of γ-aminobutyric acid type A (GABAA) receptor subtypes (α1-3;5). It has also been used as a GABAA receptor partial agonist in the subcutaneous Alzet minipumps to treat obese agouti related protein (AgRP) ablated and lean naive mice to study its effect on them.
Therapeutic Function
Anxiolytic
Biological Activity
Bretazenil is a positive allosteric modulator of GABAA receptors with anticonvulsant and anxiolytic activity. It potentiates GABA-gated chloride currents in rat cortical neurons and in HEK293 cells expressing α1β1γ2 subunit-containing GABAA receptors (EC50s = 60 and 10 nM, respectively). Bretazenil inhibits binding of the benzodiazepine diazepam to rat cerebral cortex homogenates (IC50 = 2.2 nM). It inhibits tonic convulsions induced by pentylenetetrazol (PTZ; Item No. 18682) and maximal electroshock (MES) in rats (ED50s = 0.07 and 0.48 mg/kg, respectively). Bretazenil (5-30 mg/kg) increases the number of open arm entries and percentage of time spent in the open arms of the elevated plus maze in mice, indicating anxiolytic-like activity.
Pharmacology
Bretazenil exhibits an ticonflflict and anticonvulsant properties. Only mild sedation appears at doses needed to produce anticonvulsant or anxiolytic effects, and potentiation of ethanol-induced sedation is less pronounced than with diazepam.
storage
Desiccate at +4°C
Properties of Bretazenil
| Boiling point: | 594.3±50.0 °C(Predicted) |
| Density | 1.56±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: 24 mg/mL, soluble |
| form | solid |
| pka | 0.78±0.20(Predicted) |
| color | white |
| InChI | InChI=1S/C19H20BrN3O3/c1-19(2,3)26-18(25)15-16-13-8-5-9-22(13)17(24)14-11(20)6-4-7-12(14)23(16)10-21-15/h4,6-7,10,13H,5,8-9H2,1-3H3/t13-/m0/s1 |
Safety information for Bretazenil
Computed Descriptors for Bretazenil
| InChIKey | LWUDDYHYYNNIQI-ZDUSSCGKSA-N |
| SMILES | N12C=NC(C(OC(C)(C)C)=O)=C1[C@]1([H])CCCN1C(=O)C1=C(Br)C=CC=C12 |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Bretazenil >95% CAS 84379-13-5View Details
Bretazenil >95% CAS 84379-13-5View Details
84379-13-5 -
 Bretazenil CAS 84379-13-5View Details
Bretazenil CAS 84379-13-5View Details
84379-13-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1