Flumazenil
Synonym(s):Flumazenil - CAS 78755-81-4 - Calbiochem;GABA A Receptor Antagonist, Flumazenil, Ro 15-1788;GABAA Receptor Antagonist, Flumazenil, Ro 15-1788;Ro 15-1788;Ro15-1788
- CAS NO.:78755-81-4
- Empirical Formula: C15H14FN3O3
- Molecular Weight: 303.29
- MDL number: MFCD00242764
- EINECS: 616-650-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
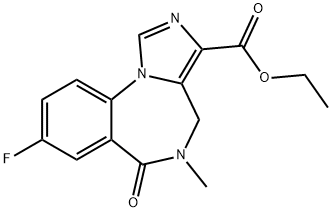
What is Flumazenil?
Toxicity
In clinical studies, most adverse reactions to flumazenil were an extension of the pharmacologic effects of the drug in reversing benzodiazepine effects.
Description
Flumazenil is a benzodiazepine antagonist useful as a fast-acting antidote in the treatment of benzodiazepine intoxication, and in reversing the central sedative effects of benzodiazepines during anesthesia.
Chemical properties
Flumazenil is a white to off-white crystalline compound with an octanol:buffer partition coefficient of 14 to 1 at pH 7.4. It is insoluble in water but slightly soluble in acidic aqueous solutions.
Originator
Hoffmann-La Roche (Switzerland)
The Uses of Flumazenil
Flumazenil is an imidazodiazepine which selectively blocks the central effects of classic benzodiazepines. It is used as benzodiazepine antagonist sedation reversal drug.
Background
Fumazenil is an imidazobenzodiazepine derivative and a potent benzodiazepine receptor antagonist that competitively inhibits the activity at the benzodiazepine recognition site on the GABA/benzodiazepine receptor complex, thereby reversing the effects of benzodiazepine on the central nervous system.
Indications
For the complete or partial reversal of the sedative effects of benzodiazepines in cases where general anesthesia has been induced and/or maintained with benzodiazepines, and where sedation has been produced with benzodiazepines for diagnostic and therapeutic procedures. Also for the management of benzodiazepine overdose as an adjunct for appropriate supportive and symptomatic measures.
What are the applications of Application
Flumazenil (Ro 15-1788) is a benzodiazepine antagonist
Definition
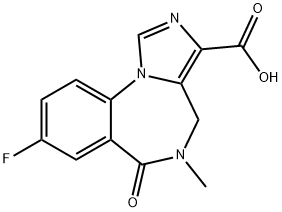
ChEBI: Flumazenil is an organic heterotricyclic compound that is 5,6-dihydro-4H-imidazo[1,5-a][1,4]benzodiazepine which is substituted at positions 3, 5, 6, and 8 by ethoxycarbonyl, methyl, oxo, and fluoro groups, respectively. It is used as an antidote to benzodiazepine overdose. It has a role as a GABA antagonist and an antidote to benzodiazepine poisoning. It is an ethyl ester, an organofluorine compound and an imidazobenzodiazepine.
Preparation
The Synthesis of Flumazenil
Starting with 4-fluoroaniline (15) the isatin 17 is synthesized via the Sandmeyer synthesis; isatin is then oxidized with peracetic acid to the isatoic anhydride 18. Reaction with sarcosine in DMF leads to the benzodiazepine-2,5-dione 19. This is converted to the iminochloride by reaction with POCI3 . In the key step the imidazoester is built up by reaction with deprotonated ethyl isocyanoacetate [8]. Since ethyl isocyanoacetate is not very stable, an alternative synthesis based on the synthesis of midazolam was developed for large scale-production. Tnthis synthesis diethylmalonate is used. The diester 21 is then transformed to the monoester 22 hy deethoxycarbonylation. Nitrosation and catalytic reduction lead to the amino compound 23. The final carbon atom is introduced by reaction with the orthoester.
brand name
Romazicon (Roche);Anexate.
Therapeutic Function
Benzodiazepine receptor antagonist, Anticonvulsant
Biological Activity
Flumazenil is a GABAA receptor antagonist with non-selective for α 1, α 2, α 3 or α 5 (IC50 = 2 nM in a radioligand binding assay using rat cortical synaptosomes). Flumazenil also acts as a partial agonist of GABAA receptors, decreasing the amplitude of electrically stimulated population spikes in rat hippocampal CA1 pyramidal neurons. It increases the number of entries into the open arms of the elevated plus maze in high-anxiety BALB/c, but not C57BL/6, mice when administered at doses ranging from 0.1 to 1,000 μg/kg. Flumazenil (5 and 10 mg/kg) prevents a reduction in burying behavior induced by the GABAA receptor positive allosteric modulator allopregnanolone in ovariectomized rats when administered at doses of 5 and 10 mg/kg. Formulations containing flumazenil have been used to reverse sedation induced by benzodiazepines and in the treatment of benzodiazepine overdose or withdrawal.
Pharmacokinetics
Flumazenil antagonizes the CNS effects produced by benzodiazepines, but does not antagonize the central nervous system effects of drugs affecting GABA-ergic neurons by means other than the benzodiazepine receptor (including ethanol, barbiturates, or general anesthetics) and does not reverse the effects of opioids.
Pharmacokinetics
Flumazenil is a competitive antagonist at the GA BAA benzodiazepine binding site for all other ligands. I t rapidly reverses the CN S and dangerous physiological effects of benzodiazepines following iatrogenic overdose or deliberate self-harm. I t has no effect on benzodiazepine metabolism. Flumazenil is rapidly cleared from plasma and metabolised by the liver and has a very short elimination half-life (<1h). Its duration of action depends on the dose administered and the duration of action of the drug to be antagonised; repeated administration or infusions may be necessary.
Clinical Use
Reversal of sedative effects of benzodiazepines in anaesthetic, intensive care, and diagnostic procedures
Veterinary Drugs and Treatments
Flumazenil may be useful for the reversal of benzodiazepine effects after either therapeutic use or overdoses. Flumazenil may be of benefit in the treatment of encephalopathy in patients with severe hepatic failure.
Metabolism
Hepatic. Flumazenil is completely (99%) metabolized. The major metabolites of flumazenil identified in urine are the de-ethylated free acid and its glucuronide conjugate.
Metabolism
Flumazenil is extensively metabolised in the liver. The carboxylic acid metabolite is the main metabolite in plasma (free form) and urine (free form and its glucuronide). This main metabolite showed no benzodiazepine agonist or antagonist activity in pharmacological tests.Flumazenil is almost completely (99%) eliminated by non-renal routes. Practically no unchanged flumazenil is excreted in the urine, suggesting complete metabolic degradation of the drug. Elimination of radiolabelled drug is essentially complete within 72 hours, with 90-95% of the radioactivity appearing in urine and 5-10% in the faeces.
Storage
+4°C (desiccate)
Mode of action
Flumazenil, an imidazobenzodiazepine derivative, antagonizes the actions of benzodiazepines on the central nervous system. Flumazenil competitively inhibits the activity at the benzodiazepine recognition site on the GABA/benzodiazepine receptor complex. In animal experiments the effects of compounds showing no affinity for the benzodiazepine receptor, e.g. barbiturates, ethanol, meprobamate, GABA mimetics, adenosine receptor agonists and other agents were not affected by flumazenil, but those of nonbenzodiazepine agonists of benzodiazepine receptors, such as cyclopyrrolones (e.g. zopiclone) and triazolopyridazines were blocked.
References
Flumazenil in benzodiazepine overdose
DOI:10.1503/cmaj.160357
Pharmacological uses of flumazenil in benzodiazepine use disorders: a systematic review of limited data
DOI:10.1177/0269881120981390
Properties of Flumazenil
| Melting point: | 201-203°C |
| Boiling point: | 528.0±50.0 °C(Predicted) |
| Density | 1.39±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Soluble in DMSO to 25mM |
| form | solid |
| pka | 0.86±0.20(Predicted) |
| color | white |
| Water Solubility | 128 mg/L |
| Merck | 14,4135 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 78755-81-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Flumazenil(78755-81-4) |
Safety information for Flumazenil
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H303:Acute toxicity,oral |
| Precautionary Statement Codes |
P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P403:Store in a well-ventilated place. |
Computed Descriptors for Flumazenil
Flumazenil manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![4H-Imidazo[1,5-a][1,4]benzodiazepine-3-carboxylicacid, 5,6-dihydro-8-hydroxy-5-methyl-6-oxo-, ethyl ester](https://img.chemicalbook.in/CAS2/GIF/131666-45-0.gif)
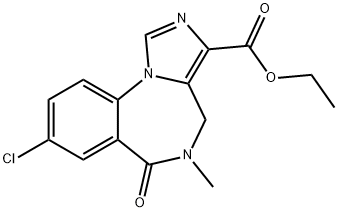
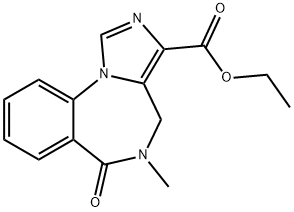
You may like
-
 Flumazenil CAS 78755-81-4View Details
Flumazenil CAS 78755-81-4View Details
78755-81-4 -
 Flumazenil 98% CAS 78755-81-4View Details
Flumazenil 98% CAS 78755-81-4View Details
78755-81-4 -
 Flumazenil CAS 78755-81-4View Details
Flumazenil CAS 78755-81-4View Details
78755-81-4 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
