L(-)-Epinephrine
Synonym(s):(−)-Adrenalin;(−)-Epinephrine;(R)-(−)-3,4-Dihydroxy-α-(methylaminomethyl)benzyl alcohol;L -Adrenaline;L -Epinephrine
- CAS NO.:51-43-4
- Empirical Formula: C9H13NO3
- Molecular Weight: 183.2
- MDL number: MFCD00002204
- EINECS: 200-098-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
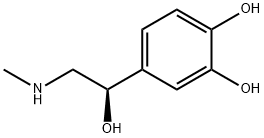
What is L(-)-Epinephrine?
Absorption
Following I.V. (intravenous) injection, epinephrine disappears rapidly from the blood stream. Subcutaneously or I.M. (intramuscular) administered epinephrine has a rapid onset and short duration of action. Subcutaneous (SC) administration during asthmatic attacks may produce bronchodilation within 5 to 10 minutes, and maximal effects may occur within 20 minutes. The drug becomes fixed in the tissues rapidly , .
Toxicity
Skin, LD50 = 62 mg/kg (rat)
Pregnancy
Epinephrine is teratogenic in rats when given in doses about 25 times the human doses. It is unknown whether epinephrine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Epinephrine should be given to a pregnant woman only if clearly required in critical situations/emergencies .
Labor and Delivery
Parenteral administration of epinephrine, if used as support for blood pressure during low or other spinal anesthesia for delivery, can lead to the acceleration of fetal heart rate and should not be used in obstetrics when maternal blood pressure is higher than 130/80. Epinephrine may delay the second stage of labour.
Common and generalized adverse effects:
Transient and minor side effects of anxiety, headache, fear, and palpitations may occur with therapeutic doses of epinephrine, especially in hyperthyroid individuals. Repeated local injections may result in necrosis at sites of injection due to vascular constriction. Cerebral hemorrhage; hemiplegia; subarachnoid hemorrhage; anginal pain in patients with angina pectoris; anxiety; restlessness; throbbing headache; tremor; weakness; dizziness; pallor; respiratory difficulty; palpitation; apprehensiveness; sweating; nausea; vomiting .
Cardiovascular effects: Inadvertently induced high arterial blood pressure may result in angina pectoris (especially when coronary insufficiency is present), cardiac ischemia, or aortic rupture , . Epinephrine may cause serious cardiac arrhythmias in patients not suffering from heart disease and patients with organic heart disease receiving drugs that sensitize the cardiac muscle. With the injection of epinephrine 1:1,000, a paradoxical but transient lowering of blood pressure, bradycardia and apnea may occur immediately post-injection .
Cerebrovascular hemorrhage: Overdosage or accidental I.V. injection of epinephrine may lead to cerebrovascular hemorrhage resulting from the sharp rise in blood pressure .
Renal vasoconstriction: Parenterally administered epinephrine initially may produce constriction of renal blood vessels and decrease urine formation. High doses may cause complete renal shutdown .
Pulmonary edema: Fatality may also result from pulmonary edema due to the peripheral constriction and cardiac stimulation produced by epinephrine injection .
Digital vasoconstriction: Since epinephrine is a strong vasoconstrictor, accidental injection into the digits, hands or feet may lead to the loss of blood flow to the affected area. Treatment should be directed at vasodilation in addition to further treatment of anaphylaxis .
Description
The combination of the catechol nucleus, the β-hydroxyl group, and the N-methyl give EPI a direct action and a strong affinity for all adrenergic receptors. Epinephrine and all other catechols are chemically susceptible to oxygen and other oxidizing agents, especially in the presence of bases and light, quickly decomposing to inactive quinones. Therefore, all catechol drugs are stabilized with antioxidants and dispensed in air-tight amber containers.
Chemical properties
off-white powder
Originator
Adrenalin,Sopharma,Bulgaria
The Uses of L(-)-Epinephrine
Endogenous catcholamine with combined α-and β-agonist activity. Principal sympathomimetic hormone produced by the adrenal medulla. Bronchodilator; cardiostimulant; mydriatic; antiglaucoma.
The Uses of L(-)-Epinephrine
L-Adrenaline (Epinephrine) belongs to a group of the compounds known as catecholamines, which play an important role in the regulation of physiological process in living organisms. The antioxidant activity and antioxidant mechanism of L-adrenaline was cla
The Uses of L(-)-Epinephrine
Gibberellic Acid-3 (GA-3) is a plant growth regulator
Background
Epinephrine, also known as adrenaline, is a hormone and neurotransmitter and produced by the adrenal glands that can also be used as a drug due to its various important functions. Though it has long been used in the treatment of hypersensitivity reactions, epinephrine in the auto-injector form (EpiPen) has been available since 1987 in the USA. Many new products/biosimilars and dosage routes have been approved under various names over the last several decades , , . On August 16, 2018, Teva Pharmaceuticals USA gained approval to market its generic epinephrine auto-injector in 0.3 mg and 0.15 mg strengths . Dosage delivery routes for epinephrine include intravenous, inhalation, nebulization, intramuscular injection, and subcutaneous injection.
In general, the most common uses of parenteral epinephrine are to relieve respiratory distress due to bronchospasm, to provide rapid relief of hypersensitivity (anaphylactic or anaphylactoid) reactions to drugs, animal serums and other allergens, and to prolong the action of infiltration anesthetics . In addition to the above functions, epinephrine is the primary drug administered during cardiopulmonary resuscitation (CPR) to reverse cardiac arrest , . It can be used in severe cases of croup .
Indications
Epinephrine injection is indicated in the emergency treatment of type I allergic reactions, including anaphylaxis. It is also used to increase mean arterial blood pressure in adult patients with hypotension associated with septic shock.
Epinephrine's cardiac effects may be of use in restoring cardiac rhythm in cardiac arrest due to various causes but is not used in cardiac failure or in hemorrhagic, traumatic, or cardiogenic shock .
Epinephrine is used as a hemostatic agent. It is also used in treating mucosal congestion of hay fever, rhinitis, and acute sinusitis; to relieve bronchial asthmatic paroxysms; in syncope due to complete heart block or carotid sinus hypersensitivity; for symptomatic relief of serum sickness, urticaria, angioneurotic edema; for resuscitation in cardiac arrest following anesthetic accidents; in simple (open angle) glaucoma; for relaxation of uterine musculature and to inhibit uterine contractions. Epinephrine injection can be utilized to prolong the action of local anesthetics .
In addition to the above, epinephrine is used as an over the counter (OTC) agent for the intermittent symptoms of asthma, such as wheezing, tightness of chest and shortness of breath . It is also used for the maintenance of mydriasis during intraocular surgery .
What are the applications of Application
(?)-Epinephrine is an adrenoceptor activator
Definition
ChEBI: The R-enantiomer of adrenaline. It is a hormone secreted by the adrenal glands resulting in the 'fight-or-flight' response.
Production Methods
Epinephrine is synthesized in the body from the nonessential amino acid tyrosine. Tyrosineundergoes hydroxylation to produce DOPA (3,4-dihydroxyphenylalanine). DOPA decarboxylationproduces dopamine, which is hydroxylated to norepinephrine. Norepinephrine, whichis closely related to epinephrine, performs a number of similar functions in the body. The prefix “nor” associated with a compound is used to denote an alkylated nitrogen in the compoundthat has lost an alkyl group. It comes from the German N-ohne-radical, which means Nitrogenwithout the radical. Therefore norepinephrine is epinephrine minus the methyl, CH3, radicalon the nitrogen. The methylation of norepinephrine gives epinephrine.
Indications
Epinephrine administered subcutaneously is used to manage severe acute episodes of bronchospasm and status asthmaticus. In addition to its bronchodilator activity through β-adrenoceptor stimulation, a portion of the therapeutic utility of epinephrine in these acute settings may be due to a reduction in pulmonary edema as a result of pulmonary vasoconstriction, the latter effect resulting from α-adrenoceptor stimulation.
Manufacturing Process
1 part by weight of ω-chloro-3,4-dihydroxyacteophenone in 1 part by weight of ethanol was heated with 60% aqueous solution of methylamine. The crystal of 3,4-dihydroxy-ω-methylaminoacteophenone obtained was transformed in hydrochloride by action of diluted hydrochloric acid. The base of (-)-3,4- dihydroxy-ω-methylaminoacteophenone was prepared by addition of ammonium hydroxide solution.
brand name
Bronkaid (Bayer); Epipen (Meridian); Primatene (Wyeth); Sus-Phrine (Forest); Twinject (Verus);Adrefil;Adrehinal;Adrenalin medihal;Adrenalina ace.p.d.;Adrenalina clorhi;Adrenalina delta;Adrenalina fustery;Adrenalina hormona;Adrenalina p davis;Adrenalina wiener;Bronkaid mistometer;Cetanest;D epinefrin;Dento-caine;Depinefrin;Dysne-inhal;E-caprine;Epiboran ofteno;Epinephrine pediatric;Epineramine;Glaucadrine;Glaucoaicon;Glauconin;Glaucotahil;Isopto epinefrina;Levoreninl-adrenaline;Licothionil;Lidoacton;Lyodrin;Marcaom;Methylaminoethanolcatechol;Neo-rybarex;Niphridine;Orostat;P2e1;Paranephrine;Piladren;Sedo-asmol;Suprarenine;Suprexon 5;Susphrine;Vaponephrine;Xylestesin a.
Therapeutic Function
Vasoconstrictor
World Health Organization (WHO)
Epinephrine, first isolated in 1899, is the main hormone secreted by the adrenal medulla. It is widely used as a vasoconstrictor substance and in the treatment of anaphylactic shock. Its use in combination with local anaesthetics to prolong infiltration anaesthesia has been associated with systemic reactions including serious cardiovascular and cerebrovascular incidents. Regulations restricting the concentrations permitted in such preparations have been introduced in many countries but combination products containing epinephrine or levarterenol in concentrations of 1:80,000 or less remain widely available. Representative preparations are included in the WHO Model List of Essential Drugs. (Reference: (WHTAC1) The Use of Essential Drugs, 2nd Report of the WHO Expert Committee, 722, , 1985)
Biological Functions
Epinephrine is found only in very low concentrations in the mammalian CNS, and it is unlikely to play a major role as a neurotransmitter.
General Description
White to nearly-white microcrystalline powder or granules. Odorless. Melting point 211-212°C. Aqueous solutions are slightly alkaline. Slightly bitter, numbing taste.
General Description
Epinephrine (E, Adrenalin) differs from NE only bythe addition of an N-methyl group. Like the other CAs, E islight sensitive and easily oxidized on exposure to air becauseof the catechol ring system. The development of apink-to-brown color indicates oxidative breakdown. Tominimize oxidation, solutions of the drug are stabilized bythe addition of reducing agents such as sodium bisulfite. Eis also destroyed readily in alkaline solutions and by metals(e.g., Cu, Fe, Zn) and weak oxidizing agents. It is used inaqueous solution for inhalation as the free amine. Like otheramines, it forms salts with acids, hydrochloride, and thebitartrate being the most common.
Like NE, it lacks oral activity and has short DOA.However, it is much more widely used clinically than NE.E is a potent stimulant of all α1-,α2-,β1-,β2-, and β3-adrenoceptors, and thus it switches on all possible adrenergicreceptors, leading to a whole range of desired and sideeffects. Particularly prominent are the actions on the heartand on vascular and other smooth muscle. It is a very potentvasoconstrictor and cardiac stimulant. NE has, in general,greater β-activity caused by an additional N-methyl group.Therefore, E is used to stimulate the heart in cardiac arrest.Although intravenous infusion of E has pronounced effectson the cardiovascular system, its use in the treatment ofheart block or circulatory collapse is limited because of itstendency to induce cardiac arrhythmias.
Air & Water Reactions
L(-)-Epinephrine darkens slowly on exposure to air and light. Water insoluble. Readily soluble in aqueous solutions of inorganic acids. Solutions undergo oxidation in the presence of oxygen.
Reactivity Profile
L(-)-Epinephrine is incompatible with oxidizers, alkalis, copper, iron, silver, zinc and other metals; gum and tannin. L(-)-Epinephrine is also incompatible with acids, acid chlorides and acid anhydrides. L(-)-Epinephrine reacts with salts of sulfurous acid .
Fire Hazard
Flash point data for L(-)-Epinephrine are not available. L(-)-Epinephrine is probably combustible.
Biochem/physiol Actions
Adrenoceptor agonist.
Mechanism of action
The effects on pulmonary function are quite rapid, with peak effects occurring within 5 to 15 minutes. Measurable improvement in pulmonary function is maintained for up to 4 hours.The characteristic cardiovascular effects seen at therapeutic doses of epinephrine include increased heart rate, increased cardiac output, increased stroke volume, an elevation of systolic pressure and decrease in diastolic pressure, and a decrease in systemic vascular resistance. The cardiovascular response to epinephrine represents the algebraic sum of both α- and β-adrenoceptor stimulation. A decrease in diastolic blood pressure and a decrease in systemic vascular resistance are reflections of vasodilation, a β2-adrenoceptor response. The increase in heart rate and systolic pressure is the result of either a direct effect of epinephrine on the myocardium, primarily a β1 effect, or a reflex action provoked by a decrease in peripheral resistance, mean arterial pressure, or both. Overt α-adrenoceptor effects, such as systemic vasoconstriction, are not obvious unless large doses are used.
Pharmacokinetics
Epinephrine is a sympathomimetic drug. It causes an adrenergic receptive mechanism on effector cells and mimics all actions of the sympathetic nervous system except those on the facial arteries and sweat glands .
Important effects of epinephrine include increased heart rate, myocardial contractility, and renin release via beta-1 receptors. Beta-2 effects produce bronchodilation which may be useful as an adjunct treatment of asthma exacerbations as well as vasodilation, tocolysis, and increased aqueous humor production . In croup, nebulized epinephrine is associated with both clinically and statistically significant transient reduction of croup symptoms 30 minutes post-treatment . Epinephrine also alleviates pruritus, urticaria, and angioedema and may be helpful in relieving gastrointestinal and genitourinary symptoms associated with anaphylaxis because of its relaxing effects on the smooth muscle of the stomach, intestine, uterus, and urinary bladder .
Clinical Use
The ability of epinephrine to stimulate β2-receptors hasled to its use by injection and by inhalation to relaxbronchial smooth muscle in asthma and in anaphylacticreactions. Several OTC preparations (e.g., Primatene, Bronkaid)used for treating bronchial asthma use E. It is also usedin inhibiting uterine contraction. Because of its α-activity, Eis used to treat hypotensive crises and nasal congestion, toenhance the activity of local anesthetics, and as a constrictorin hemorrhage.
In addition, E is used in the treatment of open-angle glaucoma,where it apparently reduces intraocular pressure byincreasing the rate of outflow of aqueous humor from theanterior chamber of the eye. The irritation often experiencedon instillation of E into the eye has led to the developmentof other preparations of the drug that potentially are not asirritating. One such example is dipivefrin.
Clinical Use
Epinephrine is used in a variety of clinical situations, and although concern has been expressed about the use of epinephrine in asthma, it is still used extensively for the management of acute attacks.
Side Effects
Patients treated with recommended dosages of epinephrine will complain of feeling nervous or anxious. Some will have tremor of the hand or upper extremity and many will complain of palpitations. Epinephrine is dangerous if recommended dosages are exceeded or if the drug is used in patients with coronary artery disease, arrhythmias, or hypertension. The inappropriate use of epinephrine has resulted in extreme hypertension and cerebrovascular accidents, pulmonary edema, angina, and ventricular arrhythmias, including ventricular fibrillation. At recommended dosages, adverse effects from inhaled isoproterenol are infrequent and not serious. When excessive dosages are used, tachycardia, dizziness, and nervousness may occur, and some patients may have arrhythmias.
Safety Profile
Human poison by subcutaneous route. Experimental poison by ingestion, skin contact, subcutaneous, intraperitoneal, intravenous, and intramuscular routes. Human systemic effects: cardiomyopathy includmg infarction, arrhythmias. An experimental teratogen. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx. Used as an adrenergic, sympathomimetic, vasoconstrictor, bronchodilator, and cardiac stimulant.
Synthesis
Epinephrine, L-1-(3,4-dihydroxyphenyl)-2-methylaminoethanol (11.1.2), is
obtained from the adrenal glands tissue of livestock [1,2] as well as in a synthetic manner.
Epinephrine is synthesized from ω-chloro-3,4-dihydroxyacetophenone—chloroacetylpyrocatechine—the reaction of which with excess of methylamine gives ω-methylamino-3,4-
dihydroxyacetophenone (11.1.1). Reduction of this using hydrogen over Raney nickel, or
action of aluminum amalgam, or electrolytic reduction gives D,L-epinephrine (11.1.2) [3–9],
which is separated into isomers using (+) tartaric acid .
Veterinary Drugs and Treatments
Epinephrine is employed primarily in veterinary medicine as a treatment for anaphylaxis or cardiac resuscitation. Because of its vasoconstrictive properties, epinephrine is added to local anesthetics to retard systemic absorption and prolong effect.
Drug interactions
Potentially hazardous interactions with other drugs
Alpha-blockers: avoid with tolazoline.
Anaesthetics: increased risk of arrhythmias if given
with volatile anaesthetics.
Antidepressants: increased risk of arrhythmias and
hypertension if given with tricyclics; MAOIs and
moclobemide may cause hypertensive crisis.
Beta-blockers: increased risk of severe hypertension
and bradycardia.
Clonidine: possible increased risk of hypertension.
Dopaminergics: effects possibly increased by
entacapone; avoid concomitant use with rasagiline.
Guanethidine: increased risk of hypertension.
Sympathomimetics: effects possibly enhanced by
dopexamine.
Metabolism
Epinephrine is rapidly inactivated mainly by enzymic transformation to metanephrine or normetanephrine, either of which is then conjugated and excreted in the urine in the form of both sulfates and glucuronides. Either sequence results in the formation of 3-methoxy-4- hydroxy-mandelic acid(vanillylmandelic acid, VMA) which is shown to be detectable in the urine . Epinephrine is rapidly inactivated in the body mostly by the enzymes COMT (catechol-O-methyltransferase) and MAO (monoamine oxidase). The liver is abundant in the above enzymes, and is a primary, although not essential, tissue in the degradation process .
Metabolism
Epinephrine is ultimately metabolized by COMT and MAO to 3-methoxy-4-hydroxy-mandelic acid (vanillylmandelic acid), which is excreted as the sulfate or glucuronide in the urine. Only a very small amount is excreted unchanged.
Purification Methods
L-Adrenaline has been recrystallised from EtOH/AcOH/NH3 [Jensen J Am Chem Soc 57 1765 1935]. Itis sparingly soluble in H2O, readily in acidic or basic solutions but insoluble in aqueous NH3, alkali carbonate solutions, EtOH, CHCl3, Et2O or Me2CO. It has also been purified by dissolving in dilute aqueous acid, then precipitating it by adding dilute aqueous ammonia or alkali carbonates. It is readily oxidised in air and turns brown on exposure to light and air. (Epinephrine readily oxidises in neutral alkaline solution. This can be diminished if a little sulfite is added). Store it in the dark under N2. [Lewis Br J Pharmacol Chemother 9 488 1954]. The hydrogen oxalate salt has m 191-192o(dec, evacuated capillary) after recrystallisation from H2O or EtOH [Pickholz J Chem Soc 928 1945]. [Beilstein 13 H 830, 13 III/IV 2927.]
Toxicity evaluation
Epinephrine is available in nebulized racemic dosage form for inhalation.Intoxication from catecholamine usually results from iatrogenic overdoses, accidental intravenous administration, and the injection of solution intended for nebulization. High concentrations of dopamine present inside of a cell than there are vesicles to store it in, oxidative stress can occur and cause damage or death to the cell. It is thought that dopamine overload causes biochemical damage to cellular mitochondria, that provide the cell with all of the energy it requires to function, resulting in death of the cell. Catecholamines produced circulatory changes that reversed propofol anesthesia in animal models.
Properties of L(-)-Epinephrine
| Melting point: | 215 °C (dec.)(lit.) |
| Boiling point: | 316.88°C (rough estimate) |
| alpha | -51.5 º (c=4, 1M HCl, dry sub) |
| Density | 1.1967 (rough estimate) |
| refractive index | -51.5 ° (C=4, 1mol/L HCl) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, in ethanol (96 per cent) and in methylene chloride. It dissolves in hydrochloric acid. |
| form | Fine Crystalline Powder |
| pka | 8.66(at 25℃) |
| color | White to light beige |
| Water Solubility | <0.01 g/100 mL at 18 ºC |
| Sensitive | Air & Light Sensitive |
| Decomposition | > 212°C |
| Merck | 14,3619 |
| BRN | 2368277 |
| Stability: | Stable. Incompatible with acids, acid chlorides, acid anhydrides, oxidizing agents. Light sensitive. |
| CAS DataBase Reference | 51-43-4(CAS DataBase Reference) |
| NIST Chemistry Reference | L-adrenaline(51-43-4) |
| EPA Substance Registry System | Epinephrine (51-43-4) |
Safety information for L(-)-Epinephrine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H310:Acute toxicity,dermal |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P262:Do not get in eyes, on skin, or on clothing. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for L(-)-Epinephrine
L(-)-Epinephrine manufacturer
Apex Healthcare Limited
S D Fine Chem Limited
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 51-43-4 Adrenaline 98%View Details
51-43-4 Adrenaline 98%View Details
51-43-4 -
 51-43-4 98%View Details
51-43-4 98%View Details
51-43-4 -
 L-Adrenaline CAS 51-43-4View Details
L-Adrenaline CAS 51-43-4View Details
51-43-4 -
 L-Adrenaline 98% (HPLC) CAS 51-43-4View Details
L-Adrenaline 98% (HPLC) CAS 51-43-4View Details
51-43-4 -
 L-Adrenaline, GR 99% CAS 51-43-4View Details
L-Adrenaline, GR 99% CAS 51-43-4View Details
51-43-4 -
 Adrenaline 51-43-4 99%View Details
Adrenaline 51-43-4 99%View Details
51-43-4 -
 L-ADRENALINE AR CAS 51-43-4View Details
L-ADRENALINE AR CAS 51-43-4View Details
51-43-4 -
 (−)-Epinephrine CAS 51-43-4View Details
(−)-Epinephrine CAS 51-43-4View Details
51-43-4