Boron trifluoride-methanol solution
Synonym(s):Boron trifluoride;Boron trifluoride-methanol solution;Trifluoroborane
- CAS NO.:373-57-9
- Empirical Formula: CH3BF3O
- Molecular Weight: 98.84
- MDL number: MFCD00071635
- EINECS: 206-766-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
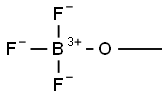
What is Boron trifluoride-methanol solution?
Chemical properties
colorless to amber solution
Chemical properties
Boron trifluoride etherates: (compounded with methyl ether) is moisture-sensitive, corrosive, flammable liquid.
The Uses of Boron trifluoride-methanol solution
It was used in transmethylation of 1,2-Diacylglycerol (DAG) containing fractions which were purified using HPLC. It may be used to treat lipids during GC analysis done for determination of fatty acid methyl esters and dimethylacetals.
The Uses of Boron trifluoride-methanol solution
Trifluoro(methanol)boron is a reagent used for the GC analysis of ritalinic acid.
The Uses of Boron trifluoride-methanol solution
Reagent for the GC analysis of ritalinic acid. Alternative to Cl2CHCO2H for synthesis of purine rich oligomers.
Lewis acid used in the preparation of geminal bis-peroxides from ketals and enol ethers with t-butyl hydroperoxide. Reagent used for the mild deacetylation of amines.
What are the applications of Application
Boron trifluoride-methanol solution is a reagent used for the GC analysis of ritalinic acid
General Description
Boron trifluoride-methanol (BF-M) is an esterification reagent. BF-M is a powerful acidic catalyst for the esterification of the fatty acids. It easily cleaves acetylated dyes resulting in free amino groups. BF-M catalyzed trans esterification of vegetable oils forms biodiesel.
Potential Exposure
Used as a catalyst.
Shipping
Diethyl: UN2604 Boron trifluoride diethyl etherate, Hazard class: 8; Labels: 8—Corrosive material, 3—Flammable liquid. Dimethyl: UN2965 Boron trifluoride dimethyl etherate, Hazard class: 4.3; Labels: 4.3— Dangerous when wet, 8—Corrosive material, 3— Flammable liquid.
Incompatibilities
Reacts with air forming corrosive hydrogen fluoride vapors. Incompatible with oxidizers (may cause fire and explosion), water, steam or heat, forming corrosive and flammable vapors. Peroxide containing etherate reacts explosively with aluminum lithium hydride, magnesium tetrahydroaluminate. Mixtures with phenol react explosively with 1,3-butadiene. Presumed to form explosive peroxides.
Properties of Boron trifluoride-methanol solution
| Melting point: | -93℃ (Methanol) |
| Boiling point: | 59 °C4 mm Hg |
| Density | 1.203 g/mL at 25 °C |
| vapor pressure | 19.5 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 52 °F |
| storage temp. | 2-8°C |
| form | clear liquid |
| color | Colorless to Light yellow |
| Specific Gravity | 0.890 (20/4℃) |
| Merck | 14,1349 |
| BRN | 3611499 |
| CAS DataBase Reference | 373-57-9 |
| EPA Substance Registry System | Boron, trifluoro(methanol)-, (T-4)- (373-57-9) |
Safety information for Boron trifluoride-methanol solution
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H314:Skin corrosion/irritation H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H370:Specific target organ toxicity, single exposure H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Boron trifluoride-methanol solution
| InChIKey | FQZVTMBNNNQKJY-UHFFFAOYSA-N |
Boron trifluoride-methanol solution manufacturer
Anand Agencies
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
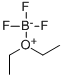
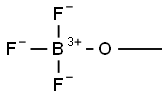
You may like
-
 Boron trifluoride-methanol complex 50-52% w/w boron trifluoride CAS 373-57-9View Details
Boron trifluoride-methanol complex 50-52% w/w boron trifluoride CAS 373-57-9View Details
373-57-9 -
![Boron Trifluoride - Methanol Reagent (10-20%) [for Esterification] (1mL×10) CAS 373-57-9](https://img.chemicalbook.in//Content/image/CP5.jpg) Boron Trifluoride - Methanol Reagent (10-20%) [for Esterification] (1mL×10) CAS 373-57-9View Details
Boron Trifluoride - Methanol Reagent (10-20%) [for Esterification] (1mL×10) CAS 373-57-9View Details
373-57-9 -
 Boron Trifluoride in Methanol (14%) Solution CAS 373-57-9View Details
Boron Trifluoride in Methanol (14%) Solution CAS 373-57-9View Details
373-57-9 -
 Boron trifluoride-methanol solution CAS 373-57-9View Details
Boron trifluoride-methanol solution CAS 373-57-9View Details
373-57-9 -
 Boron trifluoride-methanol solution CAS 373-57-9View Details
Boron trifluoride-methanol solution CAS 373-57-9View Details
373-57-9 -
 Boron trifluoride-methanol solution CAS 373-57-9View Details
Boron trifluoride-methanol solution CAS 373-57-9View Details
373-57-9 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1