Boron trifluoride diethyl etherate
Synonym(s):Boron trifluoride ethyl etherate;Boron trifluoride-diethyl ether complex;Boron trifluoride-ethyl etherate
- CAS NO.:109-63-7
- Empirical Formula: C4H10BF3O
- Molecular Weight: 141.93
- MDL number: MFCD00013194
- EINECS: 203-689-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
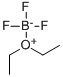
What is Boron trifluoride diethyl etherate?
Description
Boron trifluoride diethyl etherate (BF3-OEt2) is a convenient, easy to handle source of boron trifluoride (BF3). BF3-OEt2 is a liquid, whereas BF3 is a gas.
Chemical properties
Boron trifluoride etherates: (compounded with methyl ether) is moisture-sensitive, corrosive, flammable liquid.
Physical properties
Fuming liquid; stable at ambient temperatures but hydrolyzed on exposure to moist air; density 1.125 g/mL; refractive index 1.348; solidifies at -60.4°C; boils at 125.7°C; flash point (open cup) 147°F (68.8°C); decomposes in water.
The Uses of Boron trifluoride diethyl etherate
Boron trifluoride diethyl etherate is used as a Lewis acid catalyst in Mukaiyama aldol addition, alkylation, acetylation, isomerization, dehydrations and condensation reactions. It is involved in the prepattion of polyethers in polymerization reactions. As a catalyst, it is used in the preparation of cyclopentyl- and cycloheptyl[b]indoles and other diborane. It is also used in sensitive neutron detectors in ionization chambers as well as monitoring radiation levels in earth?s atmosphere.
The Uses of Boron trifluoride diethyl etherate

A solution of the SM (300 mg, 0.493 mmol) in DCM (7.5 mL) was cooled to 0 C and treated with BF3-OEt2 (0.243 mL, 1.97 mmol). The reaction mixture was stirred at 0 C for 2 h, then RT for 2 h. Additional BF3-OEt2 (0.122 mL, 0.986 mmol) was added and the mixture was stirred another 1 h. The reaction mixture was quenched with sat aq NaHCO3 and then concentrated in vacuo. MeOH was added and the mixture was stirred at RT for 24 h. The mixture was diluted with H2O and extracted with DCM (2x). The combined organics were dried (MgSO4) and concentrated in vacuo to give a white residue. The residue was purified by Prep LC (40 g silica, 0-10% MeOH/DCM) to give 141 mg of a white solid. The solid was dissolved in THF (10 mL) and 1N NaOH (1 mL) and stirred at RT for 17 h. The mixture was diluted with H2O and extracted with 5% MeOH/DCM. The combined organics were dried (MgSO4) and concentrated to give 75 mg of a white solid. The solid was purified by Prep LC (12 silica, 0-10% MeOH/DCM) to provide the product as a white solid. [64 mg, 27%]
The Uses of Boron trifluoride diethyl etherate
Catalyst in acetylation, alkylation, polymerization, dehydration, and condensation reactions.
Preparation
Boron trifluoride etherate is prepared by the reaction of vapors of boron trifluoride with that of anhydrous diethyl ether:
BF3 (g) + (C2H5)2O (g) → (C2H5)2O?BF3.
What are the applications of Application
Catalyst in the synthesis of polyol chains. Reagent for the coupling of imines to allylstannanes and 4′-nitrobenzenesulfenanilide to alkenes and alkynes.
Lewis acid reagent with broad application
Catalyst used in the preparation of cyclopentyl- and cycloheptyl[b]indoles from aryl cyclopropyl ketones via [3+2] cycloaddition.
General Description
Boron trifluoride etherate is a fuming liquid. Boron trifluoride etherate may be corrosive to skin, eyes and mucous membranes. Boron trifluoride etherate may be toxic by inhalation. Upon exposure to water Boron trifluoride etherate may emit flammable and corrosive vapors. Boron trifluoride etherate is used as a catalyst in chemical reactions.
Air & Water Reactions
Highly flammable. Fuming liquid, immediately hydrolyzed by moisture in air to form hydrogen fluoride [Merck 11th ed. 1989].
Reactivity Profile
Boron trifluoride diethyl ether complex is a stable, highly flammable, colourless to brown fuming, corrosive liquid with a sharp pungent odour. It forms explosive peroxides in contact with air or oxygen. It reacts exothermically with water to form extremely flammable diethyl ether and toxic, corrosive boron trifluoride hydrates. The chemical is incompatible with bases, amines, and alkali metals. It immediately gets hydrolysed by moisture in air to form hydrogen fluoride. Boron trifluoride diethyl ether has applications in chemical laboratory as a catalyst in chemical reactions.
Hazard
The compound is highly toxic by inhalation. Skin contact causes burns.
Health Hazard
May cause toxic effects if inhaled or ingested/swallowed. Contact with substance may cause severe burns to skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Flammable
Potential Exposure
Used as a catalyst.
Shipping
Diethyl: UN2604 Boron trifluoride diethyl etherate, Hazard class: 8; Labels: 8—Corrosive material, 3—Flammable liquid. Dimethyl: UN2965 Boron trifluoride dimethyl etherate, Hazard class: 4.3; Labels: 4.3— Dangerous when wet, 8—Corrosive material, 3— Flammable liquid.
Incompatibilities
Reacts with air forming corrosive hydrogen fluoride vapors. Incompatible with oxidizers (may cause fire and explosion), water, steam or heat, forming corrosive and flammable vapors. Peroxide containing etherate reacts explosively with aluminum lithium hydride, magnesium tetrahydroaluminate. Mixtures with phenol react explosively with 1,3-butadiene. Presumed to form explosive peroxides.
Properties of Boron trifluoride diethyl etherate
| Melting point: | −58 °C(lit.) |
| Boiling point: | 126-129 °C(lit.) |
| Density | 1.15 g/mL(lit.) |
| vapor density | 4.9 (vs air) |
| vapor pressure | 4.2 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 118 °F |
| storage temp. | Store below +30°C. |
| solubility | Miscible with ether and alcohol. |
| appearance | Light yellow liquid |
| form | liquid |
| color | brown |
| Specific Gravity | 1.126 (20/4℃) |
| explosive limit | 5.1-18.2%(V) |
| Water Solubility | Reacts |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| Merck | 14,1350 |
| BRN | 3909607 |
| Exposure limits | ACGIH: TWA 0.1 ppm; Ceiling 0.7 ppm |
| Stability: | Stable. Highly flammable. May form explosive peroxides in contact with air or oxygen. Reacts exothermically with water to form extremely flammable diethyl ether and toxic, corrosive boron trifluoride hydrates. Also incompatible with bases, amines, alkali metals. |
| CAS DataBase Reference | 109-63-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Boron trifluoride etherate(109-63-7) |
| EPA Substance Registry System | Boron, trifluoro[1,1'-oxybis[ethane]]-, (T-4)- (109-63-7) |
Safety information for Boron trifluoride diethyl etherate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H226:Flammable liquids H302:Acute toxicity,oral H314:Skin corrosion/irritation H330:Acute toxicity,inhalation H372:Specific target organ toxicity, repeated exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Boron trifluoride diethyl etherate
| InChIKey | MZTVMRUDEFSYGQ-UHFFFAOYSA-N |
Boron trifluoride diethyl etherate manufacturer
JSK Chemicals
Jaiswal S Cyber Shop
G C Int'L
ASM Organics
New Products
tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-Arg-OH HCl H2O Indole Methyl Resin 2-CTC Resin Gabapentin EP Impurity A 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 1,3-Diphenylurea 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID N-METHYL INDAZOLE-3-CARBOXYLIC ACID 2-Hydroxy-5-nitroacetophenone 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2-HYDROXY BENZYL CYANIDE DIETHYL AMINOMALONATE HYDROCHLORIDE 5-BROMO-2CYANO PYRIDINE Boldenone Clarithromycin Ethinyl Estradiol StanozololRelated products of tetrahydrofuran


You may like
-
 BORON TRIFLUORIDE ETHERATE 99%View Details
BORON TRIFLUORIDE ETHERATE 99%View Details -
 Boron trifluoride etherate 98%View Details
Boron trifluoride etherate 98%View Details -
 Boron trifluoride diethyl etherate CAS 109-63-7View Details
Boron trifluoride diethyl etherate CAS 109-63-7View Details
109-63-7 -
 Boron trifluoride diethyl etherate CAS 109-63-7View Details
Boron trifluoride diethyl etherate CAS 109-63-7View Details
109-63-7 -
 Boron Trifluoride Etherate CASView Details
Boron Trifluoride Etherate CASView Details -
 Boron Trifluoride EtherateView Details
Boron Trifluoride EtherateView Details
109-63-7 -
 Boron Trifluoride Diethyl EtherView Details
Boron Trifluoride Diethyl EtherView Details
109-63-7 -
 Boron Trifluoride EtherateView Details
Boron Trifluoride EtherateView Details
109-63-7
