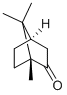
Borneol
Synonym(s):(1S)-(-)-Borneol;L-Borneol
- CAS NO.:507-70-0
- Empirical Formula: C10H18O
- Molecular Weight: 154.25
- MDL number: MFCD00003759
- EINECS: 208-080-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
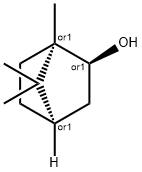
What is Borneol ?
Description
l-Borneolum was originally from the Compositae Aenean Blumea balsamifera (L.)
DC (Ai Na Xiang). Its crystals were made after being extracted from the fresh leaves
of Ai Na Xiang. It is an optical isomer with natural borneol. It’s usually used for
making spice the same as natural borneol, synthesized borneol, and so on .
Ai Na Xiang is a traditional Chinese medicine alias as Da Feng Ai, Niu Er Ai,
etc. It has been recorded firstly in Guang Zhi, “Ai Na Xiang came from the west
country and looks like the artemisia.” It has been recorded in many Chinese materia
medica books, such as Hai Yao Ben Cao, Ling Nan Cai Yaolu, etc. In Zhong hua Ben
cao (Chinese Materia Medica), the literature described it as “Ai Na Xiang
(l-borneolum) has the fragrance because it is like the artemisia. It can also be prepared to the borneol, so it is called “Bing Pian Ai.” It is believed that this is the earliest records that Ai Na Xiang was made into borneol . In China, the authentic
product area of l-borneolum is in Luodian, Guizhou Province . There are no other
plant resources reported for producing l-borneolum in China.
Chemical properties
Borneol is a bicyclic terpene alcohol. Borneol is an endo isomer;
the corresponding exo isomer is isoborneol:
Borneol occurs abundantly in nature as a single enantiomer or, less frequently, as
the racemate. (1S,2R,4S)-(?)-Borneol occurs particularly in oils from
Pinaceae species and in citronella oil. (1R,2S,4R)-(+)-Borneol is found,
for example, in camphor oil (Ho-Sho oil), rosemary, lavender, and olibanum oils.
Borneol is a colorless, crystalline solid. (+)-Borneol has a camphoraceous odor,
with a slightly sharp, earthy, peppery note, which is less evident in (?)-borneol.
Commercial borneol is often levorotatory ([α]20
D ?18 to ?28 in ethanol) and contains
(?)-borneol and up to 40% isoborneol.
Borneol is oxidized to camphor with chromic or nitric acid; dehydration with
dilute acids yields camphene. Borneol is readily esterified with acids, but on
an industrial scale, bornyl esters are prepared by other routes. For example,
levorotatory borneol is synthesized industrially from levorotatory pinenes by
Wagner–Meerwein rearrangement with dilute acid, followed by hydrolysis of the
resulting esters.
Borneol is used in the reconstitution of the essential oils in which it occurs
naturally.
Chemical properties
Borneol has a piney, camphor-like odor and burning taste somewhat reminiscent of mint.
Physical properties
Appearance: white translucent flakes, bulk or granular crystals. With a clear aroma,
spicy, cool, volatile, black smoke, and yellow flame when lit without residue left.
Melting point: 201–205? °C.? Soluble in ethanol, chloroform, or ether, almost insoluble in water. Specific optical rotation: ?36.5° to ?38.5° in 5% (g/mL) ethanol
solution, stable under sealed condition at room temperature.
Pungent, bitter, slightly cold; heart, spleen, and lung meridians entered. (In
Chinese traditional medicine’s opinion)
Occurrence
Unlike isoborneol, free or esterified borneol has been identified in more than 250 distillates from plants, herbs, leaves or bark; Compositae, Ericaceae, Lauraceae, Labiatea, Rutaceae; natural borneol can be d or l rotatory, but very seldom also racemic; most frequently encountered is the l-borneol characteristic of Compositae, Graminaceae and almost all Pinaceae; d-borneol characteristic of Cupressaceae, Zingiberaceae, lavender, lavandin and spike oils. Reported found in citrus peel oils (orange, lemon, lime), cinnamon leaf, cassia leaf, ginger, coriander seed, laurel, Ocimum basilicum, Thymus vulgaris L. and Curcuma aeruginosa Roxb.
History
l-Borneolum is one of the few drugs used from ancient times which is a single organic small molecule. With the progress of technology in modern times, it was found that there are optical differences by measuring the polarimetry, and accordingly the borneol is divided into three drugs: natural borneol (d-borneol), ai pian (l-borneol), and artificial borneol (synthetic borneol or dl-borneol).
The researches of l-borneolum are inextricably linked to borneol, but compared with the borneol, l-borneolum still has significant disadvantages. For example, borneol is used in the wider region than 1-borneolum which is mainly used in China. The pharmacological studies of borneol in modern times are much more than l-borneolum as well. The plant resources of borneol are the Cinnamomum camphora, known as the “woody incense,” and the plant resources of l-borneolum are Ai Na Xiang, known as “herbal fragrance.” From the Chinese traditional habits, it is thought that woody incense is better than herbal fragrance, so l-borneolum’s usage is limited. The purification process of borneol was done earlier than l-borneolum, and the process is excellent, with less camphor, isosorbide, and other toxic components. l-Borneolum has less purification process due to the small distribution range. Until the 1970s, l-borneolum was produced using the traditional manual process, leading to the low rate of borneol and high toxic components, finally affecting its clinical usage. In the 1980s and 1990s of the twentieth century, with the reduction of plant resources of natural borneol, the increasing demands of borneol and the poor synthesis process of borneol, the plant cultivation and purification studies of l-borneolum were developed for a while. However, with the continuous discovery of plant resources of natural borneol and the improvement of synthesis process, the research of l-borneolum was back to normal.
The Uses of Borneol
Perfumery, esters.
What are the applications of Application
Borneol is originally isolated from various aromatic plants
Definition
ChEBI: A bornane monoterpenoid that is 1,7,7-trimethylbicyclo[2.2.1]heptane substituted by a hydroxy group at position 2.
Indications
Sore throat, aphthous, red eyes, purulent ear discharge, convulsions, febrile delirium, sudden faint due to qi depression, stroke, and coma
Aroma threshold values
Detection: 140 ppb
General Description
A white colored lump-solid with a sharp camphor-like odor. Burns readily. Slightly denser than water and insoluble in water. Used to make perfumes.
Air & Water Reactions
Flammable. Insoluble in water.
Reactivity Profile
Borneol is an alcohol. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides.
Hazard
Fire risk in presence of open flame.
Health Hazard
Fire may produce irritating and/or toxic gases. Contact may cause burns to skin and eyes. Contact with molten substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.
Fire Hazard
Flammable/combustible material. May be ignited by friction, heat, sparks or flames. Some may burn rapidly with flare burning effect. Powders, dusts, shavings, borings, turnings or cuttings may explode or burn with explosive violence. Substance may be transported in a molten form at a temperature that may be above its flash point. May re-ignite after fire is extinguished.
Pharmacology
The main effects of l-borneolum are to induce resuscitation (with aromatic stimulation), clear stagnated fire (fever feeling), remove nebula to improve eyesight, and
relieve swelling and pain. In traditional Chinese medicine, borneol is often used as
an envoy drug and combined with other drugs.
The modern pharmacological researches showed that l-borneolum can cross the
blood-brain barrier (BBB) through increasing cell membrane fluidity, Na+ -K+-
ATPase activity, decreasing membrane potential, and regulating intracellular calcium concentration, which is involved in the effect of resuscitation .
The key brain-protective mechanism of l-borneolum and synthetic borneol is
closely related to the regulation of P-glycoprotein pathway, lipid peroxidation, and
nitric oxide pathway. In addition, it can regulate the calcium pathway, which is the
main biological mechanism of “Xin-floating-heart” medicinal property of borneol
and resuscitation. Based on the statistical results of strength integral law, it demonstrated that the brain-protective effect of l-borneolum is stronger than synthetic borneol, suggesting that we should prefer l-borneolum for the treatment of
cerebrovascular disease .
Clinical Use
l-Borneolum is used as a kind of borneol in clinical usage all along, but its clinical usage is fewer than borneol considering its clinical efficacy is weaker than borneol. The main reasons may be the following aspects: the discovery of l-borneolum is later than borneol, and the processing technology of l-borneolum is far behind borneol which leads to high impurities. l-Borneolum has less adverse reactions the same as borneol, including gastrointestinal irritation, reproductive toxicity, and allergic reactions.
Synthesis
Racemic borneol is prepared synthetically by reduction of camphor or from pinene.
Properties of Borneol
| Melting point: | 206-209 °C |
| Boiling point: | 210 °C(lit.) |
| alpha | -0.5~+0.5゜(20℃/D)(c=5,C2H5OH) |
| Density | 1.011 |
| vapor density | 5.31 (vs air) |
| vapor pressure | 33.5 mm Hg ( 25 °C) |
| refractive index | 1.4831 (estimate) |
| FEMA | 2157 | BORNEOL |
| Flash point: | 150 °F |
| solubility | DMSO:30.0(Max Conc. mg/mL);194.49(Max Conc. mM) Ethanol:30.0(Max Conc. mg/mL);194.49(Max Conc. mM) |
| pka | 15.36±0.60(Predicted) |
| form | powder to crystal |
| color | White to Almost white |
| Odor | at 10.00 % in dipropylene glycol. pine woody camphor balsamic |
| Water Solubility | insoluble |
| Merck | 14,1338 |
| JECFA Number | 1385 |
| Stability: | Stable. Highly flammable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 507-70-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Borneol(507-70-0) |
| EPA Substance Registry System | Borneol (507-70-0) |
Safety information for Borneol
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H228:Flammable solids H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P501:Dispose of contents/container to..… |
Computed Descriptors for Borneol
| InChIKey | DTGKSKDOIYIVQL-WEDXCCLWSA-N |
Borneol manufacturer
Indenta Chemicals (India) Pvt Ltd
Aamirav Ingredients And Specialties Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 507-70-0 Borneol 98%View Details
507-70-0 Borneol 98%View Details
507-70-0 -
 507-70-0 98%View Details
507-70-0 98%View Details
507-70-0 -
 Borneol 98%View Details
Borneol 98%View Details
507-70-0 -
 Borneol CAS 507-70-0View Details
Borneol CAS 507-70-0View Details
507-70-0 -
 Borneol (contains ca. 20% Isoborneol) CAS 507-70-0View Details
Borneol (contains ca. 20% Isoborneol) CAS 507-70-0View Details
507-70-0 -
 Borneol CASView Details
Borneol CASView Details -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1