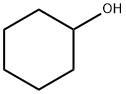
DL-Menthol
Synonym(s):(−)-Menthol;(1R,2S,5R)-2-Isopropyl-5-methylcyclohexanol;2-Isopropyl-5-methylcyclohexanol;5-Methyl-2-(1-methylethyl)cyclohexanol;Hexahydrothymol
- CAS NO.:1490-04-6
- Empirical Formula: C10H20O
- Molecular Weight: 156.27
- MDL number: MFCD00001484
- EINECS: 216-074-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-21 14:41:52
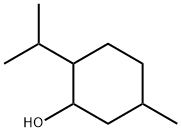
What is DL-Menthol?
Chemical properties
colourless crystals
Chemical properties
Racemic menthol is a mixture of equal parts of the (1R,2S,5R)- and (1S,2R,5S)-isomers of menthol. It is a free-flowing or agglomerated crystalline powder, or colorless, prismatic, or acicular shiny crystals, or hexagonal or fused masses with a strong characteristic odor and taste. The crystalline form may change with time owing to sublimation within a closed vessel. The USP 32 specifies that menthol may be either naturally occurring l-menthol or syntheti-cally prepared racemic or dl-menthol. However, the JP XV and PhEur 6.0, along with other pharmacopeias, include two separate monographs for racemic and l-menthol.
The Uses of DL-Menthol
Labelled Menthol. Used for oral gel patch or film containing herb extracts or Chinese medicine, fruit extract, spearmint, and menthol for smoking cessation.
The Uses of DL-Menthol
menthol is a fragrance. It is also said to be anti-septic, cooling, refreshing, and a blood-circulation stimulant. Menthol gives the skin a “cool” feeling after use. It constitutes almost 50 percent of peppermint oil but can also be synthetically produced through the hydrogenation of thymol. It is non-toxic in low doses, but in high concentrations it can be irritating to the skin, especially the mucous membranes.
The Uses of DL-Menthol
Menthol is used in confectionery, perfumery, cough drops, cigarettes, liqueurs, etc.; topical antipruritic; local anesthetic; gastric sedative.
Definition
ChEBI: Any secondary alcohol that is one of the eight possible diastereoisomers of 5-methyl-2-(propan-2-yl)cyclohexan-1-ol.
Production Methods
Menthol occurs widely in nature as l-menthol and is the principal
component of peppermint and cornmint oils obtained from the
Mentha piperita and Mentha arvensis species. Commercially, lmenthol
is mainly produced by extraction from these volatile oils. It
may also be prepared by partial or total synthetic methods.
Racemic menthol is prepared synthetically via a number of
routes, e.g. by hydrogenation of thymol.
Definition
A white crystalline terpenealcohol, C10H19OH; r.d. 0.89;m.p. 42°C; b.p. 103–104°C. It has aminty taste and is found in certainessential oils (e.g. peppermint) andused as a flavouring.
brand name
Fisherman’s Friend Lozenges (Bristol-Myers Products); Therapeutic Mineral Ice (Bristol-Myers Products).
Pharmaceutical Applications
Menthol is widely used in pharmaceuticals, confectionery, and
toiletry products as a flavoring agent or odor enhancer. In addition
to its characteristic peppermint flavor, l-menthol, which occurs
naturally, also exerts a cooling or refreshing sensation that is
exploited in many topical preparations. Unlike mannitol, which
exerts a similar effect due to a negative heat of solution, l-menthol
interacts directly with the body’s coldness receptors. d-Menthol has
no cooling effect, while racemic menthol exerts an effect approximately
half that of l-menthol.
When used to flavor tablets, menthol is generally dissolved in
ethanol (95%) and sprayed onto tablet granules and not used as a
solid excipient.
Menthol has been investigated as a skin-penetration enhancer
and is also used in perfumery, tobacco products, chewing gum and
as a therapeutic agent. When applied to the skin, menthol dilates the
blood vessels, causing a sensation of coldness followed by an
analgesic effect. It relieves itching and is used in creams, lotions, and
ointments. When administered orally in small doses menthol has a
carminative action.
Safety
Almost all toxicological data for menthol relate to its use as a
therapeutic agent rather than as an excipient. Inhalation or
ingestion of large quantities can result in serious adverse reactions
such as ataxia and CNS depression,hypersensitivity reactions,
severe abdominal pain, nausea, vomiting, vertigo, drowsiness, and
coma.Although menthol is essentially nonirritant there have been
some reports of hypersensitivity following topical application.
In a Polish study approximately 1% of individuals were determined
as being sensitive to menthol.There have been reports of apnea
and instant collapse in infants after the local application of menthol
to their nostrils.
The WHO has set an acceptable daily intake of menthol at up to
0.4 mg/kg body-weight.
LD50 (rat, IM): 10.0 g/kg
LD50 (rat, oral): 3.18 g/kg
Metabolism
Not Available
storage
A formulation containing menthol 1% w/w in aqueous cream has
been reported to be stable for up to 18 months when stored at room
temperature.
Menthol should be stored in a well-closed container at a
temperature not exceeding 25°C, since it sublimes readily.
Incompatibilities
Incompatible with: butylchloral hydrate; camphor; chloral hydrate; chromium trioxide; b-naphthol; phenol; potassium permanganate; pyrogallol; resorcinol; and thymol.
Regulatory Status
Included in the FDA Inactive Ingredients Database (dental preparations, inhalations, oral aerosols, capsules, solutions, suspensions, syrups, and tablets; also topical preparations). Included in nonparenteral medicines licensed in the UK. Accepted for use in foods and confectionery as a flavoring agent of natural origin. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of DL-Menthol
| Melting point: | 34-36 °C(lit.) |
| Boiling point: | 216 °C(lit.) |
| Density | 0.89 g/mL at 25 °C(lit.) |
| vapor pressure | 0.8 mm Hg ( 20 °C) |
| FEMA | 2665 | MENTHOL RACEMIC |
| Flash point: | 200 °F |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Very soluble in ethanol (95%), chloroform, ether, fatty
oils and liquid paraffin; freely soluble in glacial acetic acid;soluble in acetone and benzene; very slightly soluble in glycerin;
practically insoluble in water. |
| pka | 15.30±0.60(Predicted) |
| form | neat |
| form | Solid |
| color | Clear Colourless |
| Odor | at 10.00 % in dipropylene glycol. cooling mentholic minty |
| JECFA Number | 427 |
| Dielectric constant | 3.2(Ambient) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 1490-04-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Menthol(1490-04-6) |
| EPA Substance Registry System | Menthol (1490-04-6) |
Safety information for DL-Menthol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for DL-Menthol
| InChIKey | NOOLISFMXDJSKH-UHFFFAOYSA-N |
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran




You may like
-
 1490-04-6 Dementholised Peppermint Oil 98%View Details
1490-04-6 Dementholised Peppermint Oil 98%View Details
1490-04-6 -
 Menthol 99%View Details
Menthol 99%View Details -
 DL-Menthol 1490-04-6 98%View Details
DL-Menthol 1490-04-6 98%View Details
1490-04-6 -
 Menthol 98%View Details
Menthol 98%View Details
89-78-1 / 15356-70-4 -
 89-78-1 / 15356-70-4 98%View Details
89-78-1 / 15356-70-4 98%View Details
89-78-1 / 15356-70-4 -
 89-78-1 / 15356-70-4 98%View Details
89-78-1 / 15356-70-4 98%View Details
89-78-1 / 15356-70-4 -
 Menthol CAS 1490-04-6View Details
Menthol CAS 1490-04-6View Details
1490-04-6 -
 DL-Menthol 1490-04-6 98%View Details
DL-Menthol 1490-04-6 98%View Details
1490-04-6
