Citronellol
Synonym(s):(±)-β-Citronellol;(S)-(-)-ß-Citronellol;(S)-(-)-3,7-Dimethyl-6-octen-1-ol, ß-Rhodinol;3,7-Dimethyl-6-octen-1-ol;beta-Citronellol
- CAS NO.:106-22-9
- Empirical Formula: C10H20O
- Molecular Weight: 156.27
- MDL number: MFCD00002935
- EINECS: 203-375-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
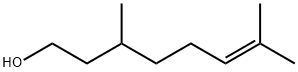
What is Citronellol?
Description
Citronellol is a kind of natural occurring acyclic monoterpenoid which can be found in citronella oils such as Cymbopogon nardus ((+)-citronellol) and rose oils and Pelargonium geraniums ((-)-citronellol). In addition to be extracted from natural oils, it can also be manufactured by the hydrogenation of geraniol or nerol. It is mainly used in perfumes and insects repellents as well as being used as a mite attractant. It should be noted that it is an excellent mosquito repellent at short distances. Combination with beta-cyclodextrin can make it has an average duration time of 1.5 hour against the mosquitoes. It can also be used for the manufacture of rose oxide. One of its most common applications is for adding floral and citrus notes to perfumes, soaps and cosmetics.
Chemical properties
colourless liquid with a characteristic, rose-like, smell
Chemical properties
Citronellol has a characteristic rose-like odor. Because odor plays such an important part in selecting this material, there may be special grades of citronellol that do not meet the Essential Oil Association specification. These limits have been broadened enough to include best qualities of commercial citronellol and chemically pure citronellol. l-Citronellol has a sweet, peach-like flavor; d-citronellol has a bitter taste.
Occurrence
l-Citronellol has been found in the plants of the Rosaceae family; d- and dl-citronellol have been identified in Verbenaceae, Labiatae, Rutaceae, Geraniaceae and others; citronellol has been reported in about 70 essential oils and in the oil of Rosa bourbonia; the Bulgarian rose oil has been reported to contain more than 50% l-citronellol, whereas East African geranium contains more than 80% of the d-isomer; the natural product is always optically active. Reported found in guava fruit, orange, bilberry, blackcurrant, nutmeg, ginger, corn mint oil (Mentha arvensis L. var. piperascens), mustard, pennyroyal oil (Mentha pulegium L.), hop oil, tea, coriander seed, cardamom, beer, rum, and apple juice.
The Uses of Citronellol
citronellol is a constituent of plant essential oils. Found abundantly in eucalyptus oil. It is used for masking odor or providing a fragrance component to a cosmetic product.
The Uses of Citronellol
Perfumery, flavoring agent.
The Uses of Citronellol
rac-Citronellol is a monoterpene found in the essential oil of various plants with antihypertensive properties. It possesses hypotensive actions due to its vasodilator abilities and is a phytochemical used in perfumes and insect repellents.
What are the applications of Application
(±)-β-Citronellol is a phytochemical used in perfumes and insect repellents
Definition
ChEBI: A monoterpenoid that is oct-6-ene substituted by a hydroxy group at position 1 and methyl groups at positions 3 and 7.
Preparation
By reduction of citronellal or geraniol or by fractional distillation of such essential oils as geranium and citronella (Bedoukian, 1967).
Aroma threshold values
Detection at 11 ppb to 2.2 ppm; l-form, 40 ppb
Taste threshold values
Taste characteristics at 20 ppm: floral, rose, sweet and green with fruity citrus nuances.
Synthesis Reference(s)
The Journal of Organic Chemistry, 60, p. 2260, 1995 DOI: 10.1021/jo00112a056
Synthesis, p. 391, 1976
Tetrahedron Letters, 30, p. 5677, 1989 DOI: 10.1016/S0040-4039(00)76168-5
General Description
Citronellol is a volatile monoterpenic primary alcohol mainly found in the essential oil of plants such as Pelargonium graveolens, Cymbopogon winterianus and Rosa damascena. It is also one of the glycosidically bound aroma compounds in ginger.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion, skin contact, and intramuscular routes. A severe skin irritant. A combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALCOHOLS.
Synthesis
It is generally accepted to distinguish rhodinol as the product isolated from geranium consisting of a mixture of l-citronellol and geraniol, whereas the name l-citronellol should be used to indicate the corresponding synthetic product with the highest level of purity; dl-citronellol can be prepared by catalytic hydrogenation of geraniol or by oxidation of allo-cyrnene; l-citronellol is prepared from (+) d-pinene via (+) cis-pinene to (+) 2,6-dimethyl-2,7-octadiene and, finally, isolating l-citronellol by hydrolysis of the aluminum-organo compound.
Purification Methods
Purify them bydistillation through a cannon packed (Ni) column and the main cut collected at 84o/14mm and redistilled. Also purify via the benzoate. [IR: Eschenazi J Org Chem 26 3072 1961, Naves Bull Soc Chim Fr 505 1951, Beilstein 1 IV 2188.]
References
https://eic.rsc.org/magnificent-molecules/citronellol/2000020.article
https://en.wikipedia.org/wiki/Citronellol
Properties of Citronellol
| Melting point: | 77-83 °C(lit.) |
| Boiling point: | 225 °C(lit.) |
| alpha | -0.3~+0.3°(D/20℃)(neat) |
| Density | 0.857 g/mL at 25 °C(lit.) |
| vapor density | 5.4 (vs air) |
| vapor pressure | ~0.02 mm Hg ( 25 °C) |
| refractive index | n |
| FEMA | 2309 | DL-CITRONELLOL |
| Flash point: | 209 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform, Methanol (Sparingly) |
| pka | 15.13±0.10(Predicted) |
| form | Liquid |
| color | Clear almost colorless |
| Odor | at 100.00 %. floral leather waxy rose bud citrus |
| Water Solubility | SLIGHTLY SOLUBLE |
| Merck | 14,2330 |
| JECFA Number | 1219 |
| BRN | 1721507 |
| Stability: | Stable. Incompatible with oxidizing agents. |
| CAS DataBase Reference | 106-22-9(CAS DataBase Reference) |
| NIST Chemistry Reference | 6-Octen-1-ol, 3,7-dimethyl-(106-22-9) |
| EPA Substance Registry System | Citronellol (106-22-9) |
Safety information for Citronellol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Citronellol
| InChIKey | QMVPMAAFGQKVCJ-UHFFFAOYSA-N |
Citronellol manufacturer
New Products
(R)-1-Boc-3-hydroxypyrrolidine Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

You may like
-
 citronellol 98%View Details
citronellol 98%View Details -
 citronellol 98%View Details
citronellol 98%View Details -
 citronellol 99%View Details
citronellol 99%View Details -
 citronellol 98%View Details
citronellol 98%View Details -
 citronellol 99%View Details
citronellol 99%View Details -
 citronellol 98%View Details
citronellol 98%View Details -
 Citronellol 97% (GC) CAS 106-22-9View Details
Citronellol 97% (GC) CAS 106-22-9View Details
106-22-9 -
 Citronellol CAS 106-22-9View Details
Citronellol CAS 106-22-9View Details
106-22-9
