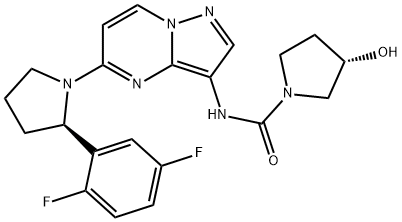
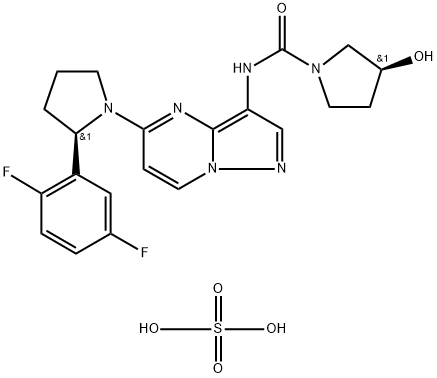
BMN 673
- CAS NO.:1207456-01-6
- Empirical Formula: C19H14F2N6O
- Molecular Weight: 380.35
- MDL number: MFCD22666357
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
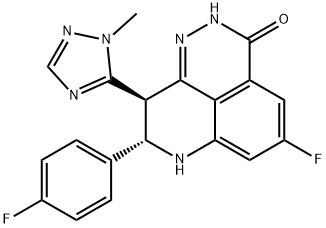
What is BMN 673?
Absorption
After administration of talazoparib 1 mg orally once daily, the mean [% coefficient of variation (CV%)] AUC and maximum observed plasma concentration (Cmax) of talazoparib at steady-state was 208 (37%) ng x hr/mL and 16.4 (32%) ng/mL, respectively. The mean (CV%) steady-state Ctrough was 3.53 (61%) ng/mL. Steady state was reached within two to three weeks of therapy. The Tmax ranges from one to two hours.
A high-fat, high-calorie food increased the mean Cmax by 46% and the median Tmax from one to four hours, without affecting the AUC.
Toxicity
There is no information available regarding the acute toxicity (LD50) of talazoparib. There is no specific treatment in the event of talazoparib overdose, and symptoms of overdose have not been established. In the event of an overdose, discontinue treatment with talazoparib, consider gastric decontamination, follow general supportive measures, and treat symptomatically.
The Uses of BMN 673
BMN 673 is a pyrrolopyrazine derivative and anpoly (ADP-ribose) polymerase (PARP) inhibitor for patients with advanced or recurrent solid tumors such as ovarian and breast cancer.
Background
Talazoparib is an inhibitor of mammalian polyadenosine 5’-diphosphoribose polymerases (PARPs), enzymes responsible for regulating essential cellular functions, such as DNA transcription and DNA repair.
Developed by Pfizer, talazoparib was first approved by the FDA in October 2018 and by the EMA in June 2019. It was approved by Health Canada in September 2020. Talazoparib is currently used in the treatment of BRCA-mutated breast cancer and HRR-mutated prostate cancer.
Indications
Talazoparib is indicated for the treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) HER2-negative locally advanced or metastatic breast cancer. This indication is approved by the FDA, EMA, and Health Canada.
In the US, talazoparib is also indicated in combination with enzalutamide for the treatment of adult patients with HRR gene-mutated metastatic castration-resistant prostate cancer (mCRPC).
Biological Activity
bmn673 is a potent and selective parp1/2 inhibitor with ki of 1.2 and 0.9 nm, respectively 1. it had no effect on panels of 72 receptors, ion channels, and enzymes 1. bmn673 showed ic50 value of 0.57 nm in enzymatic assay of parp1 1. in in vitro assay, it exhibited greater potency than other existing parp inhibitors, such as veliparib, rucaparib, and olaparib 2. it is also much more potent at trapping parp-dna complexes than other parp inhibitors 3.bmn673 has shown anti-tumor activity both in vitro and in vivo. it inhibited proliferation of tumor cells and xenografts with defects in homologous recombination 1. the combination of bmn673 and dna-damaging agents demonstrated synergistic anti-tumor effects 1. in addition, study showed that the expression levels of dna repair proteins and status of pi3k pathway predict response to bmn673 in small cell lung cancer 4.bmn673 is currently under investigation in multiple
Pharmacokinetics
Talazoparib is a cytotoxic and anti-tumour agent. In vitro, talazoparib caused cytotoxicity in cancer cell lines that harboured defects in DNA repair genes, including BRCA1 and BRCA2. Talazoparib mediated anti-tumour effects on patient-derived xenograft breast cancer models bearing mutated BRCA1 or mutated BRCA2 or wild type BRCA1 and BRCA2.
Enzyme inhibitor
This novel, orally bioavailable poly(ADP-ribose) polymerase, or PARP, inhibitor (FW = 380.35 g/mol; CAS 1207456-01-6), also known as (8S,9R)- 5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8,9-dihydro- 2H-pyrido[4,3,2-de]phthalazin-3(7H)-one, targets PARP-mediated DNA repair (IC50 = 0.58 nM) of single-strand DNA breaks by the base-excision repair pathway. By enhancing the accumulation of DNA strand breaks, BMN 673 promotes genomic instability, eventually leading to apoptosis. BMN 673 exhibits selective anti-tumor cytotoxicity and elicits DNA repair biomarkers at much lower concentrations than earlier generation PARP1/2 inhibitors, including olaparib, rucaparib and veliparib. BMN 673 shows remarkable anti-tumor activity in vivo, with strong action against xenografted tumors carrying defects in DNA repair due to BRCA mutations or PTEN deficiency. Synergistic or additive anti-tumor effects are observed, when BMN 673 was combined with temozolomide, SN38 or platinum drugs.
Metabolism
Talazoparib undergoes minimal hepatic metabolism. The metabolic pathways include mono-oxidation, dehydrogenation, cysteine conjugation of mono-desfluoro talazoparib, and glucuronide conjugation.
References
1. shen y, rehman fl, feng y et al. bmn 673, a novel and highly potent parp1/2 inhibitor for the treatment of human cancers with dna repair deficiency. clin cancer res 2013; 19: 5003-5015.2. cardnell rj, byers la. proteomic markers of dna repair and pi3k pathway activation predict response to the parp inhibitor bmn 673 in small cell lung cancer--response. clin cancer res 2014; 20: 2237.3. murai j, huang sy, renaud a et al. stereospecific parp trapping by bmn 673 and comparison with olaparib and rucaparib. mol cancer ther 2014; 13: 433-443.4. cardnell rj, feng y, diao l et al. proteomic markers of dna repair and pi3k pathway activation predict response to the parp inhibitor bmn 673 in small cell lung cancer. clin cancer res 2013; 19: 6322-6328.
Properties of BMN 673
| Melting point: | 247 - 249°C |
| Density | 1.63 |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 10.14±0.60(Predicted) |
| color | White to Off-White |
Safety information for BMN 673
Computed Descriptors for BMN 673
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4